Obesity and asthma have increased in prevalence dramatically among children and adolescents. There is strong epidemiological evidence of an association between these chronic morbidities, suggesting many pathophysiological pathways which may play a role in this association such as adipokines, oxidative stress, physical activity, comorbidities and diet.
Physical activity and exercise are recommended to reduce obesity and the risk of several diseases. However, exercise is a common trigger of asthma, known as exercise-induced bronchoconstriction. Similarly, obesity-related physical deconditioning may also lead to dyspnoea and exercise limitation which can mimic asthma symptoms.
Furthermore, to confirm that excessive weight is a risque factor for asthma, the evidence of causality must establish whether weight loss should decrease in asthma symptoms. The purpose of this review is to summarize what is known about the complex relationship between obesity, asthma and physical activity.
Overweight and Obesity are a real public health problem and the culprit behind several cardiovascular, metabolic, respiratory diseases.1 The prevalence of obesity among children and adolescents has increased dramatically in developed and developing countries and represents a serious pandemic, with more than 100 million cases (aged 2–18 years) in 2015.2
Asthma is a common lung disorder among children and adolescents, it is characterized by reversible airways obstruction, dyspnoea, wheezing, coughing, bronchial hyperresponsiveness, especially at night or early morning.3 It was reported that the variations in prevalence rates of asthma symptoms have increased in children and adolescents ranging from 11.1 to 11.6% and from 13.2 to 13.7%, respectively.4 Moreover, obesity and higher incidence and prevalence of asthma have been strongly connected in many epidemiological studies.5 Previous studies have shown the co-occurrence of obesity and asthma, which suggests an association between these disorders.6,7 However, there is a scientific controversy concerning the proposed pathophysiological pathways of the link between obesity and asthma.8
In addition, high prevalence of both conditions is probably initiated by a combination of genetic predisposition and environmental factors, such as exercise and physical activity levels, diet, sedentariness and early life exposure to allergens.9 Moreover, previous studies have suggested that physical activity has anti-inflammatory effects, but it remains unclear how such effects can alleviate the risk of developing asthma.10
The main objective of the present review article is to summarize the recent knowledge on the possible underlying pathophysiological mechanisms in obesity-related asthma. In addition, to know the impact of physical activity, aerobic capacity and weight loss on the asthma–obesity pathogenesis.
Epidemiology of obesity-related asthmaChildhood obesity has a negative effect on health, it causes a wide range of serious complications. In addition, Obese children and adolescents with asthma are more likely to have more symptoms, poor control of asthma, frequent and/or more severe exacerbations, corticosteroid resistance and reduced health-related quality of life.11
Several epidemiological studies have revealed the link between asthma and obesity and showed that obesity is a risk factor in the development of asthma.12,13 The previous studies findings provide evidence that an obese-asthma phenotype does exist. However, there is a scientific controversy about the mechanisms of the association between obesity and asthma.14 Obesity represents a risk factor for all life's stages, it has been shown that maternal obesity, in addition to raising the risk of pregnancy complications, can have a negative impact on the newborn's health. According to The Developmental Origins of Health and Disease (DOHaD) hypothesis, exposure to certain environmental factors during early life and development may have an impact on individual's health in childhood and later life.15
In other words, asthma may start in utero.16
A meta-analysis conducted by Forno et al.17 concluded that high maternal BMI in pregnancy or excessive maternal gestational weight gain were correlated with an increased high risk of childhood asthma. Interestingly, this can occur even for mothers without a history of asthma antecedent. Moreover, Mannino et al.18 in a prospective cohort study of 4393 newborn babies without a diagnosis of asthma during the first 2 years, the investigators found that elevated BMI at the age of 2 years among non-asthmatic children represents a significant risk factor for developing asthma during childhood for boys.
In examining the obese-asthma connection, it is important to assess whether this association is mediated by sex, race, or ethnicity in the paediatric population. A meta-analysis research that examined the possible effect of sex differences and pubertal status on the asthma-obesity link, among 6 prospective cohort studies involving 14,083 children, revealed that there was a dose-response effect between increasing BMI and asthma incidence. Further, obese boys had a significantly higher effect compared to obese girls (relative risk, boys: 2.47; 95% CI, 1.57–3.87; girls: 1.25; 95% CI, 0.51–3.03).19 In contrast to the previous findings, Yao et al.20 in a cohort of 12,092 children (6331 boys and 5761 girls), found a significant association between a higher BMI and asthma in boys and girls, but this relationship was strongest in girls. Similarly, it has been shown that obesity in childhood is considered to be a risk factor for developing asthma in later life adult.21
On the basis of the available evidence, Black et al.22 found that children and adolescents who are overweight, moderately or morbidly obese had higher odds of asthma. Conversely, this relationship appears to differ by race/ethnicity.
Lastly, a distinction between these opposing results may, therefore, the question of whether obesity preceded the development of asthma or children with asthma gain weight as a result of other factors such as physical inactivity or side effects of medications. However, it has been suggested that being overweight or obese precede the onset of asthma in adult women as well as in adult men.23 There is not as much evidence for children, but it is also more probable that childhood obesity preceded asthma.24
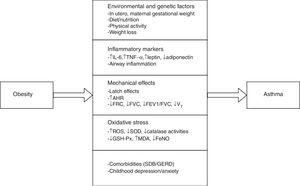
Pathophysiological mechanisms in obesity-related asthmaObesity has probably an effect on the development of asthma through various mechanisms, which could plausibly develop the disease depending on the age of exposure (including before birth), childhood, various dietaries, sex, hormone levels or cytokines, environment factors, comorbidities25 and oxidative stress26 (Fig. 1).
Pathophysiological pathways between obesity and asthma in children and adolescents. IL-6, interleukin-6; TNFα, tumour necrosis factor α; AHR, airway hyperresponsiveness; FRC, functional residual capacity; FVC, forced vital capacity; FEV1, forced expiration volume in 1 second; VT, tidal volume; ROS, reactive oxygen species; SOD, superoxide dismutase; GSH-Px, glutathione peroxidase; MDA, malondialdehyde; FeNO, exhaled nitric oxide; SDB, sleep-disordered breathing; GERD, gastroesophageal reflux disease.
The obesity–asthma relationship has been explained by several hypotheses, often controversial because this relationship appears to be multifactorial as mentioned above.
Systemic inflammation and adipokinesObesity in humans is associated with chronic low-grade systemic inflammation. Adipose tissue, in addition to its role as a storage depot of energy reserves, has important functions as an endocrine organ, producing a variety adipokines such as leptin, adiponectin, interleukin-6 (IL-6) and tumour necrosis factor-alpha (TNF-α).27 Asthma is a chronic inflammatory disease of the airways leading to bronchial hyperresponsiveness.28 Therefore, probably obesity could affect asthma by increasing airway inflammation.
The stress-related alterations in the immuno-inflammatory mechanism associated with persistent low-grade systemic inflammation from obesity activate key immune cells or the pulmonary epithelium and render an individual susceptible to additional factors such as atopy, pollutant exposure, diet and comorbidities, which lead to the development of obesity-related asthma. In addition, in children and adolescents with an underlying genetic predisposition for asthma/allergy, the onset of obesity might trigger sufficient inflammatory activation to accelerate the development of frank disease.29
One such adipokine is leptin, that plays an important role in controlling appetite, neuroendocrine function, lipid and bone metabolism. Moreover, in regulating innate and adaptive immunity.30 In addition to the functions mentioned above, leptin levels are a key factor in regulating pulmonary inflammation and bronchoconstriction in murine animal's models of obesity-related asthma.31
Studies in adults indicated that obese subjects have higher serum leptin and interleukin-6 levels in blood of obese subjects than of lean subjects. The same results were found in obese children with asthma compared with normal weight asthmatic children, associated with no differences in IL-6 and tumour necrosis factor-α (TNFα) levels.24 Moreover, Several cross-sectional studies have reported a significant positive relationship between leptin levels and asthma in normal weight asthmatic children compared to healthy controls in which leptin levels represent a predictive factor for the onset of asthma. Similarly, Multiple regression analysis showed the contribution of body mass index (BMI) and asthma in predicting leptin levels in children.
IL-6 contributes to the inflammatory process by increasing CRP levels and decreases adiponectin production. A positive correlation between adipocyte diameter and IL-6 level has been observed but not with the percentage of fat or BMI in the paediatric population. High levels of IL-6 associated with obesity may potentially contribute to increased pulmonary inflammation and asthma symptoms.
In addition, Previous research has indicated potential associations between leptin and asthma pathogenesis via vascular endothelial growth factor (VEGF) which contributes to airways remodelling and regulates angiogenesis.32,33 Leptin resistance has been identified as a contributing factor in increasing parasympathetic tone and it causes bronchoconstriction and obesity-asthma phenotype.34
Adiponectin, in contrast to other adipokines secreted by adipose tissue, appears to have anti-inflammatory effects and plays a protective role in cardiovascular diseases. According to the literature, Obese people have lower levels of adiponectin than normal-weight individuals.35 On the contrary, in another study, no significant differences have been found in leptin and adiponectin concentrations in an obese asthmatic group versus the control group.24 These findings, therefore, suggest a modulating effect of sex and age on the adipokines-asthma relationship.36
The C-reactive protein (CRP) is used as a marker of inflammation and for predicting the risk of coronary artery heart disease.37 Shin et al.38 enrolled 103 children (73 obese, 30 normal weight) between the ages of 8–15 years, they found significant differences in the plasma concentration of the C-reactive protein (CRP) between healthy weight children and obese children, while a study conducted by Huang et al.39 failed to identify a significant differences in CRP levels between obese and normal weight children with or without asthma (The principal limitation of this study is its small sample size). Furthermore, it has been found that another adipokine, resistin, may reduce the asthma risk in the paediatric population. Further research is needed to better understand the complex interaction between adipokines and asthma.
Oxidative stress, obesity and asthmaOxidative stress is defined as an imbalance between the production of pro-oxidants such as (e.g., ROS or RNS) and antioxidant defence mechanisms in the body.40 Based on the fact that obesity and asthma are inflammatory diseases characterized by high oxidative stress.41 Consequently, their combined effect contributes to producing more systemic oxidative stress, which is involved in asthma pathogenesis.42 The association between chronic inflammation and oxidative stress recruit and activate inflammatory cells such as neutrophils and eosinophils, which produces reactive oxygen species (ROS).43 Moreover, high levels of oxidative stress may increase the airway inflammation by increasing hyperresponsiveness, stimulating the mucin-secreting cells and inducing inflammatory mediators, which are interconnected with asthma severity.44 Additionally, endogenous ROS-induced injury in airway smooth muscle cells leads to their hypercontractility.45
Several natural antioxidants are present in the lungs to counteract the harmful effects of oxidants; these include enzymatic as well as nonenzymatic antioxidants. Enzymatic antioxidants include catalase, glutathione peroxidase (GSH-Px) and superoxide dismutase (SOD) and nonenzymatic antioxidants are vitamin E, vitamin C, albumin, uric acid, ceruloplasmin, and glutathione (GSH).46 Oxidant and antioxidant imbalance can also result in oxidative stress. Subject with asthma has lower concentrations of SOD and catalase activities in association with decreased lung function. Also, there is a suppressed activity of catalase, SOD, and GSH-Px in patients with asthma.47
In addition, the nonenzymatic antioxidants (i.e., vitamin C, vitamin E, reduced GSH, lycopene, and carotenoids) have also reported being low in asthmatic children.46 Furthermore, it has been shown that asthma is related to higher levels of malondialdehyde (MDA), which is the most frequent agent of oxidative stress in lung diseases, and lower levels of GSH in the intervention group versus the control group and changes in these biomarkers concentrations are associated with the severity of asthma.48
Last years, there has been an increasing interest in assessing exhaled breath condensate (EBC) in subjects with pulmonary disease. The analysis of EBC is a noninvasive method of measuring several biomarkers of airway inflammation. The safety of EBC has been demonstrated in both adults and children.49 Montuschi et al.50 found that patients with asthma have higher oxidative stress as reflected by the EBC concentration of 8-isoprostanes than their normal weight counterparts. However, it is uncertain if the increased oxidative stress is a consequence of the systemic Oxidative Stress associated with obesity.51 In addition, Zanconato et al.52 reported that children with asthma had higher plasma levels of EBC cys-LTs and 8-isoprostane, compared with healthy controls. Changes in nitric oxide (NO) metabolism has a relevant role in the obese–asthma association.14
It has been found that there is a significant inverse association between exhaled nitric oxide (FeNO) and total antioxidant capacity, and directly with the plasma level of MDA.53
Indeed, Leung et al.54 found increased levels of FeNO and Leukotriene B4 (LTB4) concentration in children with asthma. However, there were no significant differences in inflammatory markers between obese children with asthma and their non-obese counterparts.
In summary, little is known about the interrelationships between obesity, asthma and oxidative stress on the basis of some conflicting experimental findings, but it is plausibly that oxidative stress may not be a causal factor but rather may modulate asthma severity and alter the response to medications.51
The mechanical effects of obesity on respiratory function and asthmaThe mechanics of breathing depends on the interaction between the lungs, the chest wall and respiratory muscles. Obesity is another factor that has been studied as a possible contributing factor in modifying lung volume, so it has been shown that lower static lung volumes are related to obesity but spirometric lung volumes and airflow are usually within normal limits except in morbidly obese individuals.55
Obese subjects present an airway wall stiffness, which is caused by a combination of both of the effects of obesity on the lung volumes and the effort of the chest wall in breathing. In addition, Reducing lung compliance appears to be correlated with an exponential increase in BMI.56
Several methods of determining obesity have been used to describe the relationship between obesity and respiratory function, BMI has been used as an index of adiposity because it correlates well with body fat but there is conflicting data regarding the association of BMI with respiratory function, some studies have demonstrated a significant negative relationship57,58 and no significant relationship in other studies.59 On the other hand, BMI does not distinguish lean mass from fat mass and takes no account of differences in fat distribution.60
Moreover, thoracic and abdominal adiposity have a direct impact on chest wall properties and diaphragm downward movement by increasing intra-abdominal pressure. As a consequence, abdominal compartment syndrome (ACS) occurs, which leads to decreasing lung volumes and pulmonary dynamics.56 particularly in supine posture, leads to a ventilation-perfusion mismatch or V/Q defects in the posterobasal compartments of the lung, and this mismatch leads to the effect of alveolar hypoventilation.61
In adults, a high level of body mass index (BMI) is related to numerous physiological changes in lung function, regardless of asthma such as a decrease in functional residual capacity (FRC) and expiratory reserve volume (ERV). In addition, there is a moderate reduction in total lung capacity(TLC), vital lung capacity (VLC) and residual volume (RV) in obese individuals with a BMI≥30 compared to normal weight individuals.62
These alterations of lung physiology lead obese subjects to breathe shallowly near to their closing volume with a reduced tidal volume at higher respiratory rates and higher subjective perception of dyspnoea.63
In addition to the abovementioned changes, it has been reported that obesity has an alterable effect on airway smooth muscle contractility and increased airway hyperresponsiveness.36 However, There are controversial data about the relationship between obesity and airway hyperresponsiveness (AHR). According to the European Community Respiratory Health Survey, there is a positive relationship between AHR and BMI in men but not in women. In contrast, as noted by Sutherland.64 there is an association between BMI and asthma diagnosis and symptoms such as dyspnoea and wheeze, but no association was found between BMI and airflow obstruction or AHR.
Physical or mechanical effects of obesity on the respiratory system are probably to play a key role in the association between obesity and asthma in children.24 Obese children and adolescents have altered pulmonary function, resulting by reducing forced expiratory volume in one second (FEV1), forced vital capacity (FVC) and forced expiratory flow between 25% and 75% of FVC (FEF25–75%).65
Obesity is also associated with restricted tidal volume changes, particularly during exercise. Moreover, smooth muscle function will also be directly affected by restricted cyclic tidal volume. As a consequence, slowly cycling latch-bridges between actin and myosin resulting in increasing the airway smooth muscles constriction and it becomes difficult to stretch which leads to persistent airway obstruction and bronchial hyperactivity.66
Lastly, Forno et al.17 studied the relationship between adiposity indicators, asthma, and atopy in children, they found a significant association between BMI and asthma, but no association between Adiposity indexes (fat mass percentage, waist circumference and waist to hip ratio) and asthma in children. Future research should, therefore, concentrate on the investigation of the relationship between the percentage, distribution of body fat and mechanical changes.
Comorbidities of obesity and asthmaIt would appear that obesity-related disease increases the risk of developing asthma. For instance, obesity is a risk factor for sleep-disordered breathing (SDB) and for gastroesophageal reflux disease (GERD), and both GERD and SBD may increase the risk of asthma.67 It has been demonstrated that treatment of SDB with nasal continuous positive airway pressure has a significant improvement in asthma outcomes and peak expiratory flow (PEF) rates.68 Sulit et al.69 found that SDB and obesity each are linked to asthma/wheeze. The relationship between obesity and wheeze may be partly caused by factors associated with SDB.
Studies showing the coexistence of GERD and asthma, with a complex relationship between these conditions. GERD plays an important role in increasing or maintaining asthma symptoms or Symptoms of asthma may overlap those of GERD.70 Epidemiological studies report a variable prevalence of GERD in children with asthma ranging from 19.3% to 65%.71
Finally, childhood depression and anxiety are related to both asthma and obesity, and it may commonly go neglected and untreated. As a consequence, some obese children with asthma need special and high self-management education and self- help such interventions, training by care providers and coaching .the management programme for children with persistent asthma involve regular physical activity and exercise, weight management and asthma education.72
Physical activity and exercise-induced bronchoconstriction in obesity-related asthmaLifestyle factors play a key role in the pathogenesis of asthma-obesity phenotype, particularly low levels of physical activity (PA).73 It has been widely accepted that diet and physical activity (PA) play important roles in maintaining health and preventing disease. Indeed, 5 of the 6 noncommunicable diseases (NCDs) risk factors are highly associated with diet and physical activity.74 Increasing physical activity and cardiovascular fitness have a positive impact on the peak expiratory flow and well-controlled asthma,75 improve quality of life, reduce exacerbations, hospital admissions, school absenteeism, unplanned medical visits and the number of medications used in asthma control.76 The possible physiological explanations of the health benefits of physical activity in asthmatic children include; reduced mucus and oedema due to the improvement of the mucociliary clearance via increased epithelial stimulation, decreased smooth muscle latching and increased chest wall force induced by increased deep inspiration and sigh rate.77 In contrast, physical inactivity can cause airway stenosis by reducing the distensibility of the smooth muscles. A recent study found an association between increased bronchial hyperresponsiveness in asthmatic children and decreased hours of exercise per week.78
It has been reported that lower physical activity levels associated with lower aerobic fitness have appeared during the same period in which incidences of obesity and asthma were higher. In addition, The combination of obesity-asthma related inflammatory stimuli can lead to a vicious cycle in obese children with asthma.12 As obese children with asthma have lower FEV1/FVC ratio than normal weight asthmatics, it may be supposed that lower airway obstruction contributes to decreasing physical activity.79
There are controversies surrounding the relationship between asthma and physical activity. A number of studies found lower physical activity levels in children with asthma, whereas others reported no difference in physical activity levels between asthmatic children and healthy peers.80 Furthermore, physical activity and exercise limitation were significantly reduced in 30% of children with asthma.81 The principal factor may be their relative hyperventilation during exercise, which causes dyspnoea and exercise-induced bronchoconstriction (EIB).82
Previous studies have reported that normal breathlessness during exercise is interpreted as being dangerous asthma symptoms and therefore children react by avoiding physical activity.83
Exercise-induced bronchoconstriction (EIB) is the most common clinical problem in patients suffering from asthma. It is characterized by breathlessness, wheezing, coughing, and chest tightness occurring during or after exercise. Asthmatic individuals react by avoiding exercise and physical activity due to a fear of experiencing EIB symptoms may be the best explanation for the lack of aerobic fitness.84 As a result, skeletal muscle deconditioning caused by hypoactivity may play a key in decreasing physical fitness levels.82
Studies investigating EIB in children with asthma found that obese children with asthma have higher fall in forced expiratory volume in one second (FEV1) and a slower recovery from EIB compared to normal weight asthmatic children which have been identified as a limiting factor to their participation in sports activities.85 Furthermore, It has been proved that using asthma medications prior to exercise can prevent EIB. Previous evidence has reported the safety and multiple benefits of staying active for patients with asthma. However, these studies vary in terms of their design, as well as in mode, frequency, intensity and duration of physical activity.86
A longitudinal study conducted by Lochte.87 who followed a group of 28 asthmatic children and 27 healthy between the ages of 7 and 15 for ten months. All subjects performed 6min submaximal treadmill test, which consists of an exercise-induced asthma test, predicted aerobic capacity was estimated by using an equation and physical activity was assessed by a questionnaire. The results obtained showed a lower predicted aerobic capacity (PAC) values during the experimental period and physical activity was positively correlated with PAC in the intervention group. In addition, it has been reported that obese children have higher exertional perception than in age-matched controls.88
Additionally, Regular physical activity and other types of exercise are known to improve the levels of different antioxidants system, and consequently to decrease oxidative stress. These effects are shown to be related to the amount of physical activity.43
As noted above, regular exercise reduces fatness and increases the production of anti-inflammatory cytokines from contracting skeletal muscle such as myokines. Also, high levels of physical activity have been suggested to reduce and prevent asthma evolution.89,90 However, lower physical activity levels in children with asthma are not universal and seem to depend on the potential effects of parental attitudes and teaching about the benefits of exercise in asthma control and may also be related to the emotional health of children.72
Weight loss strategies and diet in obesity-related asthmaFew studies have been conducted to determine the obesity-asthma relationship differently, supposing that if obesity has a negative impact on asthma outcomes, then weight reduction must lead to decrease the asthma prevalence or at least lessen asthma symptoms or health care services. If it is correct, that would provide causal evidence on the weight–asthma relationship.91
It would seem that weight loss induced by dietary or bariatric surgery appears to be associated with significant improvement in asthma symptoms, pulmonary function and quality of life.92 A 12-month observational study was conducted by Bafadhel et al.93 included 151 adult patients diagnosed with severe refractory asthma, 74% of patients had a high BMI. At the end of the intervention period, 23% of patients lost weight and a significant correlation was found between weight loss and increased FEV1, but no significant association with disease symptom control or exacerbations. Furthermore, van Huisstede et al.94 performed a longitudinal study, aimed to study the effects of bariatric surgery on asthma control and lung function. All patients were divided into three Groups. A group of obese patients with asthma who underwent bariatric surgery, a group of obese without asthma also underwent bariatric surgery and a group of obese subjects with asthma without undergoing bariatric surgery. As a result, there is an association between surgically induced weight loss and asthma control, quality of life and lung function. Additionally, in a retrospective cohort study, Sikka et al. found a significant decrease in respiratory medication after surgically induced weight loss.95
In the first randomized controlled trial study in obese children with asthma, assessing the impact of diet-induced weight loss on asthma. The authors reported that weight loss improved static lung function and asthma control. However, no change has been observed in systemic and airways inflammation.96 In contrast, another study found an improvement in biomarkers of inflammation, lung function and asthma severity after a weight loss programme in asthmatic and healthy obese adolescents.97
More recently, Willeboordse et al.98 investigate the effect of 18 months multifactorial weight loss programme on asthma outcomes. No significant differences has been found between the experimental versus the control group. In addition, it was observed an improvement in body weight and asthma outcomes during the experimental period in both groups. While, the noticeable effect was observed in the experimental group (forced vital capacity, asthma control, and quality of life).
Several epidemiologic studies have suggested that dietary characteristics contribute to the development of asthma, such as low dietary intake of vegetables, fish, and high in saturated fats. Similarly, omega-6 fatty acids (ω-6 fatty acids) were significantly related to obesity and the onset of asthma symptoms.99 In this case, the ω-6 fatty acids may play a key role in the regulation of immune response and inflammatory process. The arachidonic acid which is synthesized from linoleic acid represents a precursor of prostaglandin, which rises inflammation and contributes to asthma development.100 Conversely, omega-3 fatty acids have anti-inflammatory effects and it has an inverse relationship with asthma incidence in young adults.101
ConclusionAs shown above, an undeniable relationship exists between obesity and asthma. More recently, several hypotheses have been proposed to explain this relationship, the exact mechanisms remain elusive and are multifactorial. It could plausible that obesity antedates asthma rather than early life asthma can lead to obesity onset. Furthermore, it has been suggested that these underlying mechanisms connected to obesity and asthma may inhibit or develop the disease depending on genetic and environmental exposure that interact with each other. Obesity-induced systemic inflammation in which adipokines play a role in creating a pro-inflammatory state combined with mechanical alterations of the airways postulated to play a key role in obesity-associated asthma. Moreover, obesity-related deconditioning may lead to dyspnoea and perceived exercise limitation inducing symptoms that could mimic asthma.
The role of decreased physical activity deserves more attention, as both obesity and asthma could lead to reduced engagement in physical activity. Exercise-induced bronchoconstriction, uncontrolled symptoms and parental overprotection may lead to a vicious cycle of further reduction in physical activity and aerobic fitness in obese children with asthma. However, Prophylactic treatment of exercise-induced bronchoconstriction prior to exercise is recommended and is reported to control symptoms. Recently, there has been growing interest in dietary and weight loss strategies, suggesting improvement in lung function, asthma control and severity, and quality of life in obese children with asthma. In addition, increased consumption of fruit, vegetables and fish, which are rich in antioxidants, may alleviate asthma symptoms.
The evidence reviewed here seems to encourage physical activity, weight loss interventions including a dietary component and exercise programme which should be recommended by health care professionals for the prevention and management of obesity-related asthma in children and adolescents.
Formal consentFor this type of study, formal consent is not required.
Ethical approvalThis article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interestA. Oudjedi and K. Said Aissa declare that they have no competing interests.