El término enfermedad tromboembólica venosa se refiere a varios procesos patológicos, entre los que destacan la trombosis venosa profunda, el tromboembolismo pulmonar, la hipertensión pulmonar tromboembólica crónica y el síndrome postrombótico. La importancia en nuestro medio reside en que es una patología que precisa un periodo de recuperación largo, de 3 a 6 meses, y que un diagnóstico tardío o no bien realizado puede ocasionar una enfermedad más grave e incluso un desenlace fatal. Es difícil establecer su prevalencia en el ámbito del deporte, aunque de forma empírica parece ser similar a la del individuo que no hace deporte. Sin embargo, el ámbito del deporte y su entorno ofrece condiciones clínicas de riesgo que pueden ser factores que precipiten su presencia, la contusión sobre el lecho vascular, el reposo de los viajes, la deshidratación, la masoterapia mal orientada, ciertas medicaciones o una predisposición genética. La presente guía ofrece una actualización del proceso, se expone la protocolización diagnóstica, las pautas de prevención y de tratamiento estándar y aplicado al deporte, pensando no solo en el deportista sino también en el profesional y en el personal acompañante.
The term venous thromboembolism refers to various pathological processes among which deep vein thrombosis, pulmonary embolism, chronic thromboembolic pulmonary hypertension and the thrombotic syndrome. The importance in sports activities is that it is a pathology that requires a long recovery period from 3 to 6 months, and a delayed or unsuccessful diagnosis can cause a more serious illness or even a fatal outcome. Its prevalence in the field of sport is difficult to establish, but empirically it seems to be similar to that of the individual who does not practice sport. However, the field of sport and its environment offers clinical risk conditions to be taken into account, bruising on the vascular bed, rest, travel, dehydration, misguided massage therapy, certain medications or a genetic predisposition, may be factors that precipitate their presence. This guide updates the process, explains the diagnostic protocol and provides prevention guidelines and general treatments, also applied to sport, thinking not only of sport but also the professional and accompanying personnel.
Definition
The term venous thromboembolic disease (VTED) covers several pathological processes including deep vein thrombosis (DVT), pulmonary thromboembolism (PTE), chronic thromboembolic pulmonary hypertension and post-thrombotic syndrome. DVT is the presence of a thrombus in a vein, accompanied by a variable inflammatory response. PTE is the formation of a thrombus inside a vein and its later embolisation in the pulmonary artery, totally or partially blocking it.
In sport, the intrinsic risk of suffering a VTED is similar to that of non-athletic individuals. However, athletes are in a situation that could result in exposure to unusual or a higher number of risk factors such as injuries, travel, immobilisation, haemoconcentration, and polycythaemia. The presence of a genetic hypercoagulability disorder adds a further risk1 which can then be extraordinarily increased in individuals who fraudulently use certain performance enhancing drugs. On the other hand, thrombophlebitis may be brought about by or expedited by various aetiopathogeneses associated with direct or indirect injury that is accompanied by inflammation of the vessel. The presence of the thrombus may be previous to or a consequence of this inflammation, always aggravating the clinical profile.
Incidence. The latest reviews indicate that the incidence of the first episode of DVT among the general population is 1.2/1,000 inhabitants/year and it affects 3-5% of the population. It is the third cause of cardiovascular death after the coronary syndrome and cerebrovascular accidents. The mortality of VTED is 14-17% at three months and that of PTE is 25% at one week. The morbidity is explained by the recurrence of 5-7% of VTED cases at three months; 20% DVT at 5 years and 23% PTE at 5 years, 17-50% post-thrombotic syndrome at one year and 23% at two years, pulmonary arterial hypertension recurrence rate of 1 to 5%2 and an incidence of severe haemorrhages of 5%3.
Aetiopathogenesis
VTED is a multifactorial and complex disease where the interaction of genetic, estimated at 60%, and environmental factors, use of contraceptives, pregnancy, immobilisation or cancer, among others, determine the risk of thrombosis for each individual4. It is important to stress that genetic factors play a very important role and the exposure to prothrombotic environmental factors will trigger appearance of the event.
The known genetic factors are deficiencies in anti-thrombin-III, protein C and protein S (natural anticoagulants of the blood coagulation cascade), Factor V Leiden mutation and the G20210A mutation in prothrombin or F-II gene. Over the last few years this list has been extended and at present there is solid scientific evidence showing the implication of other genetic disorders in the risk of thrombosis5. Based on this scientific evidence, it is essential to evaluate the presence of prothrombotic genetic factors when assessing the risk of thrombosis in an athlete. This is then especially important if they present a personal or family history of venous thromboembolic disease. The integration of clinical and genetic data provides more information for more efficient and personalised diagnosis, treatment and prevention of thromboembolic disease.
The pathogenicity and development of DVT involves three factors known as Virchow’s triad: injury to the vein wall, venous stasis and hypercoagulability. Damage to the endothelium means that it loses its capacity to inhibit coagulation and initiate the fibrinolytic process. Stasis due to immobilisation or vein obstruction inhibits the clearance and dilution of activated coagulation factors. Finally, the congenital or acquired thrombophilic conditions promote the thrombotic process6,7. The factors that may simplify the appearance of transitory DVT are shown in Table 1.
Classification
DVT of lower extremities is classified according to its location as:
Distal
Including: Calf veins (gastrocnemius), tibioperoneal trunk (posterior tibial and peroneal veins), anterior tibial veins and soleal veins.
Asymptomatic in 75% of cases.
Only 5% give rise to PTE or post-thrombotic syndrome.
Without treatment, 20-30% progress into proximal areas.
Proximal
Including: External iliac vein, internal iliac vein, common femoral vein, deep femoral, superficial femoral, and popliteal veins.
90% of PTEs are caused by emboli from proximal DVT.
Diagnosis
This is usually through risk assessment, clinical examination and complementary tests8.
Risk assessment
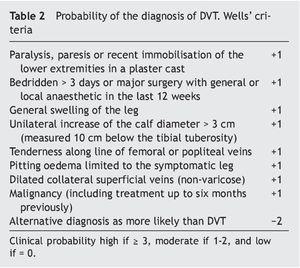
The probability of diagnosing DVT is established by the Wells model for proximal thrombosis9-11 (Table 2).
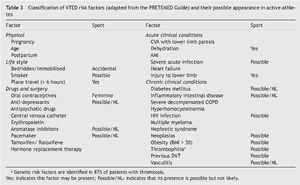
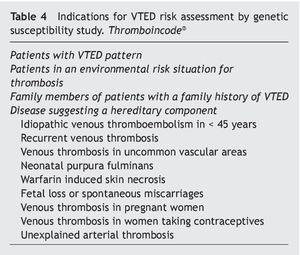
There is a classification of risk factors for active athletes that combines those intrinsic to their activity with those related to their quality of physically active individual (Table 3). The genetic risk in patients and family members with a risk of suffering thromboembolic events was studied by considering the factors mentioned in Table 45,12.
Clinical assessment
The main signs of inflammation are tumour, rubor, heat and pain. Pain is usually the first symptom. Of insidious or spontaneous onset and with a sensation of heaviness or tension in the extremity, it is often accompanied by functional difficulty. The location varies depending on the affected area. In the case of the lower limbs, it usually appears in the calf region and along deep vein pathways (hollow of the knee, Hunter’s canal, and groin). Homans’ sign (forceful dorsiflexion of the foot with the knee straight causes pain in the calf and hollow of the knee) only appears in one third of DVT cases, and more than 50% of patients do not show this sign, or it exists without the process5,13.
The oedema is initially soft and with fovea, affecting regions distal to the venous obstruction. Palpating the muscle, especially the calves, reveals a characteristic induration. This hardening should not be confused with oedema of subcutaneous cell tissue typical of other affectations not related to venous disease.
The increased superficial venous network that gradually forms as a result of a mechanism to compensate venous drainage and although it may not be appreciated in the very early phases of thrombosis, may be a clear sign in proximal or somewhat more evolved thrombosis.
As the signs and symptoms are not very specific, and may refer to other acute or chronic affections such as a torn muscle, cellulitis, lymphoedema, certain neurological processes, etc. A careful case history and examination should be worked up accompanied by other methods to simplify diagnosis.
Complementary tests
1. Echo-doppler of the lower limbs. The technique of choice for suspected proximal DVT, offering high-sensitivity and specificity in symptomatic patients. Its sensitivity is reduced in distal DVT and in asymptomatic patients, its efficacy being less than ideal in patients with distal, pelvic or recurrent thrombosis.
2. Basic analysis with blood count, biochemistry and coagulation tests determining D-Dimer. In this case, normal is considered to be below 500 ng/ml14. Studies of its power as an analytical marker of VTED concluded that it has high sensitivity (98-100%) and low specificity (35-39%)15. If this is combined with the negative predictive value that could be 98%, this test becomes a useful tool for exclusion diagnosis of DVT, but not for establishing it. For this reason it is a variable forming part of the diagnostic algorithms used at present16 but which has no value in itself. D-Dimer is exclusively generated by the degradation of stabilised fibrin, whereas fibrinogen degradation products (FDP) may originate from degradation of fibrinogen or destabilised fibrin; the latter producing monomeric D fragments but never DDimer17. For this reason, D-Dimer is a specific marker of fibrinolytic activity in processes with excessive fibrin formation. However, other pathological processes that are not thrombotic may also cause elevation of D-Dimer18. Possible false negatives. However, it has a lower predictive value of around 85% in patients with distal or infrapopliteal thrombosis, thrombosis of more than 1 week of progress (the figures may become normal), anticoagulated patients, distal thrombosis and pulmonary embolism in subsegmental arteries. Elevated D-Dimer values will never be sufficient to diagnose VTED. The D-Dimer value is also used to determine the progress of the process19,20.
3. ECG and Chest Rx.
4. Angio-magnetic resonance imaging. A non-invasive thromboembolism diagnostic method with a sensitivity and specificity comparable to phlebography in pelvic and femoral vein thrombosis, offering the possibility of a combined examination of the lower limbs and the respiratory system. Useful in patients with plaster casts, during pregnancy and in patients allergic to the iodinated contrast phlebography requires.
5. Computerised axial tomography (CAT scan). A CAT scan with contrast is also a useful test for diagnosing proximal DVT. It allows assessing the location of a previously placed vena cava filter if necessary. In the case of suspected PTE the most suitable procedure is to initially perform an angio-pulmonary CAT scan because of its proven high sensitivity in the diagnosis of pulmonary embolism compared to angiography and ventilation-perfusion scintigraphy.
6. Determination of the genetic susceptibility. This determination explains 60% of VTED cases. The presence of any of the indications shown in table 4 justifies its evaluation.
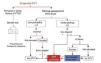
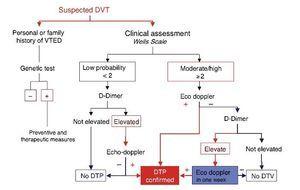
Algorithm for therapeutic DVT diagnosis (Figures 1 & 2)
Figure 1. DVT diagnostic algorithm in the athlete. The accessibility of Ultrasonographic diagnosis facilitates the application of this technique in an early stage. Sport physician must familiarize with the exploration of the vascular territory for this purpose.
Figure 2. Evaluation of suspected DVT in ambulatory medicine.
The accessibility to ultrasound scans in the field of sport enables accelerating the diagnostic process and so risk evaluation and Echo-doppler can be performed quickly in the event of clinical suspicion while requesting a D-Dimer determination. On the other hand, it may even be immediate if adequate technology is available. The presence of DDimer will only corroborate the previous diagnosis by Doppler Ultrasonography or will provide information about the progress of the process.
Echo-doppler21
This ultrasound imaging technique has become the initial and main diagnostic test for the diagnosis of DVT due to its high-sensitivity and specificity, especially in the proximal venous sector. The most direct and reliable sign is the impossibility of complete collapse of the vein walls when compressed with the echographic probe in cross-sectional projection. On occasions it is possible to directly view the texture of the intraluminal thrombus and subjectively determine the age of the thrombus by its degree of echogenicity; the higher the echogenicity, the older the thrombus.
The reliability of the echo-doppler in diagnosis of DVT when dealing with proximal venous sectors (femoral and popliteal veins and large proximal veins of the soleus and gastrocnemius) offers high sensitivity (96%) and specificity (98%). However, when the DVT is limited to the plexus soleus and gastrocnemius veins the sensitivity is reduced (73%)22. This low sensitivity in the distal sector means repeating the ultrasound scan one week later whenever it is negative and the clinical suspicion high23.
In any examination of medial and distal segments of the plexus soleus and gastrocnemius it is impossible to ensure complete collapse of the wall of each and every soleal-calf vein. This is mainly due to the small calibre of the veins at this level and the difficulty to detect complete compressibility as a direct sign of the presence of a thrombus. Under these circumstances the experience of the examiner becomes extremely important, as does the optimisation of every echo-doppler unit with slow flow rates, the systematic comparison with the asymptomatic contralateral extremity, manoeuvres to effectively increase the flow through expression of the footpad with the extremity inclined, the detection of colour irrespective of the examination angle of the echographic probe (angio or power-doppler) or the selective use of echocontrasts.
Indirect signs of normality are the existence of spontaneous flow or variation of the flow in relation to movements of the diaphragm. These are detectable by B colour mode or doppler spectrum. However, it is only possible to detect them in large diameter veins such as the femoral veins or the ileus-caval venous system. In more distal sectors it is necessary to assess the permeability of the plantar or soleal-calf venous plexus by manual compression or by using a sleeve.
The echographic signs that will help differentiate chronic and acute situations are:
• The vein does not fully collapse and it has a normal diameter, unlike what occurs in acute DVT when the diameter is larger.
• The vein walls can be seen as swollen and poorly defined.
• The thrombotic material inside the vein is not echolu-cent or homogeneous as occurs in acute DVT.
• There are often replacement venous systems in the area around the totally or partially occluded vein, with development of collateral venous circulation through unusual anatomical spaces.
• There are veins with partial occupation of their lumen showing valvular insufficiency to venous reflux challenges.
• There is an echo-doppler examination report of a previous acute episode that can be compared to the current examination.
The echo-doppler report for an examination of suspected acute DVT should indicate:
• Whether the absence of collapse of the vein wall is complete or partial.
• Whether the diameter of the vein is increased compared to the contralateral extremity.
• Define the vein sector affected by the thrombosis and its proximal and distal extension.
• Define the echographic characteristics of the thrombus (echolucent or echogenic).
However, remember that the clinical criteria of Wells’ risk stratification, together with the determination of DDimer, enable good diagnostic efficacy for DVT of the lower extremities in Emergency Rooms. In the event of low clinical probability with D-Dimer negative, the performance of Doppler Ultrasonography is unnecessary as it can be assumed to be negative for the diagnosis of DVT in the lower extremities24 (Figure 2)
Complications
Pulmonary thromboembolism
The greatest complication is a PTE and this may occur from important veins such as those in the legs, pelvis, abdomen, arms or neck. The annual incidence of PTE is 0.1%; ranging between 0.01% in young adults up to 1% in the over 60 age group, and causes de high morbidity and mortality25,26. More than half of these events originate as DVT. On the other hand, we are faced with a situation with high mortality derived from underdiagnosis. Only one of every three deaths from pulmonary embolism is diagnosed before death, and when this occurs and the condition is treated adequately, the mortality rate drops considerably. It should be taken into consideration in the event of any important bruising in these regions and appearance of symptoms indicating the process. PTE may only appear with dyspnoea and/or tachypnoea at rest or slight effort, sometimes in addition to tachycardia and in some cases chest pain and/or haemoptysis.
Recurrence of DVT
Patients who are anticoagulated for brief periods (6 to 12 weeks) for uncomplicated calf DVT have a 20% risk of progression of the thrombus to proximal DVT. The risk of recurrence is 8 to 30%, and that of developing post-thrombotic syndrome 1.8 to 20%. Long term oral anticoagulation implies a risk of severe haemorrhage of up to 5% annually (Khan 2015). This type of anticoagulation to prevent recurrence should be considered in patients with a previous history of thrombosis or congenital or acquired susceptibility.
Treatment
The treatment will depend on the diagnostic indications and combined with pharmacological anticoagulation and general measures27.
Superficial vein thrombosis or thrombophlebitis
• Hospital admission not required, although it may be recommended in recurrent cases to dismiss Trousseau’s syndrome (migratory thrombophlebitis associated with neo-plastic processes).
• Low molecular weight heparin (LMWH):
— Without affectation of the saphenous arch (Clexane 40 mg, Fragmin 5,000 IU, Hibor 3,500 IU, Innohep 4500, all every 24 hours with a minimum duration of 7-10 days.
— In the event of affectation of the saphenous arch, 3 months anticoagulation is recommended and an appointment with a haematologist.
• Analgesia.
• Relative rest.
• Use of graduated elastic compression stockings.
Confirmed deep vein thrombosis
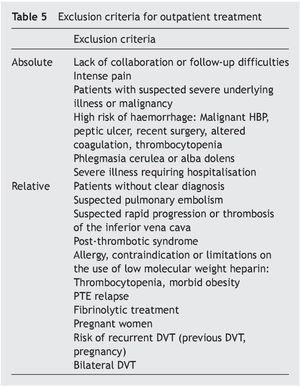
• Outpatient treatment except for the reasons shown in Tables 5-7.
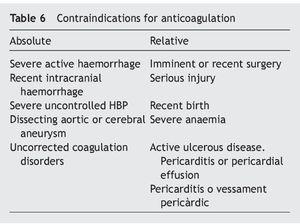
• Prescribe VKA (vitamin K antagonist) with INR therapeutic interval between 2-3; new oral anticoagulants (NOACs) or LMWH adjusted to the patient›s weight (Table 8)28,29
• Use of compression stockings to the root of the affected limb, avoiding excessive compression and varying it depending on the progress of the oedema30.
• Analgesia.
• Active mobilisation of the affected extremity should be initiated as soon as possible. Rest should be relative as of the first day of hospitalisation: although the need has not been demonstrated, it should be adjusted depending on the pain and oedema (adjust days depending on the stage of the thrombus as determined by a specialist in Echo-doppler).
• When beginning activity: (i) protect the individual from direct or indirect injuries that require a level of haemostasis within normal limits; (ii) prevent high intensity isometric exercises of the affected extremity or any that require a considerable increase diaphragm pressure; (iii) determine the degree of activity depending on the recovery process as determined by additional tests (Dimer D, thrombus quality) and the anticoagulation level and associated aspects that characterise normal physical activity. Table 9 shows the standard recommendations in this regard until considered fit to compete31.
Duration of the treatment
In patients with a transient trigger (Table 1) recurrence is lower (3%), compared to a spontaneous event (10%). Treatment over 3 months with vitamin K antagonist (VKA), NOACs or LMWH reduces the risk of recurrence by 90%. In patients with greater risk factors, such as surgery, the risk of recurrence is < 3% and so treatment can be suspended after 3 months. In moderate patients, the risk is somewhat higher (5%), and so consideration should be given to maintaining treatment up to 6 months as the risk of haemorrhage is 2% at one year.
That is, in an athlete who presents VTED treatment should be maintained for at least 3 months and consideration given to duration of up to 6 months.
Prevention
Calculation of the probability of a correct diagnosis (Table 2)
In low risk patients: No special measures.
Patients at risk of VTED: LMWH at preventive doses, or thrombin and factor Xa inhibitors or vitamin K antagonists.
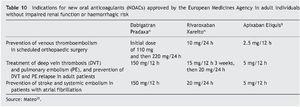
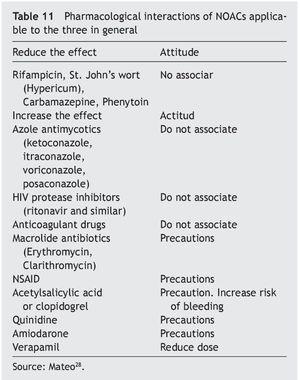
In athletes the decision to use LMWH or NOACs will depend on the experience of the medical team and the type of sport played. Table 10 shows the concepts in relation to the possible use of one or another and the interactions with certain treatments or nutrients are shown in Table 11.
For example: With NOACs, any sporting activity with an adversary or the possibility even though remote of a bruise, advise against performing this activity. With LMWH one administration at night permits morning training with guarantees, as does administration in the morning for evening training.
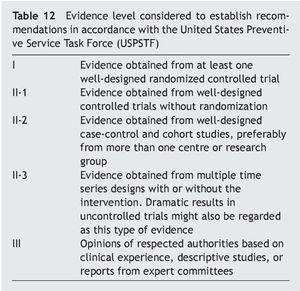
General measures and evidence level (Table 12)
Activity
Immobility increasesthe risk of DVT 10 fold32. The mobility of the muscles of the lower limb should be stimulated to prevent the formation of thrombi. Leg exercises reduce venostasis and should be recommended (Evidence I and II-1). This requires:
• Walking. When travelling by plane or train, walk for at least five minutes every hour along the aisles of the plane or carriage. When travelling by car or coach, stop every hour and take advantage of the opportunity to walk.
• If the sitting position must be maintained, an attempt should be made to activate the muscles. Bend the knees, move the feet by flexing, stretching or rotations, press the feet progressively against the floor, alternating one, the other, and both at the same time.
Hydration
Haemoconcentration increases the viscosity of the blood and reduces flow, especially in the deep veins in the legs of immobile patients33,34, good hydration should be ensured (Evidence II-3).
Mechanical methods
Mechanical methods that ensure passive mobilisation of the lower limbs, imitate muscle contractions and increase the venous flow volume and speed35.
• Graduated elastic compression stockings (GECS) (8-18 mmHg).
• Intermittent pneumatic compression (IPC) (pre- and post-surgery).
• Mechanical pumps for the feet (pre- and post-surgery).
Mechanical methods are indicated in patients with increased risk of bleeding, thus making pharmacological prevention hazardous. They are contraindicated in patients with a risk of ischaemic skin necrosis or peripheral neuropathy.
Graduated elastic compression stockings (GECS). A meta-analysis of randomised controlled studies of DVT prevention36 found that it occurred in 8.6% of patients treated compared to 27% of controls (OR 0.34; 95% CI 0.25, 0.46)12, and so GECS can be said to be effective in the prevention of DVT in surgical patients (Evidence I) with stockings up to above knee height being preferred. The effectiveness of GECS increases significantly when combined with pharmacological prevention (Evidence I).
One multicentric observational study found that the combined method is more effective that pharmacological prevention alone37 (Evidence II).
Intermittent pneumatic compression (IPC) (pre- and post-surgery). Not described in this review.
Mechanical pumps for the feet (pre- and post-surgery).
Not described in this review.
Prevention of VTED in sport
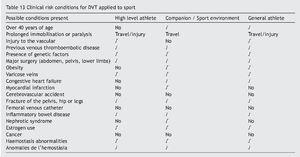
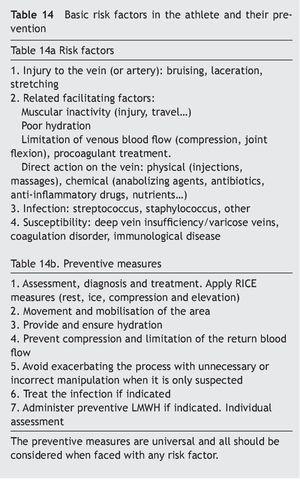
Patient. The athlete and companions with clinical conditions of risk applied to the field of sport, should include the presence of genetic factors as a risk condition (Table 13).
Consideration. The athlete may suffer important injuries that compromise venous flow to a greater or lesser degree and trigger an inflammatory process in the vessel with the consequent procoagulant alteration limiting anticoagulant activity. These affections may be of certain importance on musculotendinous tissue and blood vessels of the area, especially if they occur on the leg, ankle or foot as on many occasions they require immobilisation and a certain period of rest. Up to 34% of Achilles tendon ruptures result in DVT, irrespective of whether they are operated on or not38. In other cases the need for surgical, diagnostic or remedial tests for example in knee arthroplasty, the guidelines for surgical prevention provide limited practical orientation as, depending on the series presented, the prevalence covers a very wide range and it is not clear how to transfer the results of randomised studies into clinical practice, as a large number of cases de VTEDs are luckily limited by routine prevention when available and are asymptomatic distal DVT. Therefore, there is no consensus regarding the real application of pharmacological prevention actions, and so patients subjected to knee arthroscopy should be managed on an individual decision based on VTED risk assessment instead of a general protocol39. A recent study to assess the incidence of DVT in subjects undergoing arthroscopy for reconstruction of the ACL reported an incidence of DVT in 12% during the first week, higher than when the surgery is to repair other structures at the same time. In summary, the incidence increases by about 5% for every 30 min of tourniquet application. This means it is 5.6% if this time is < 90 min; 12.8% if between 90 and 120 min, and 17.4% if this time exceeds 120 min40.
Consideration should also be given to the fact that participating in any sport implies a certain degree of dehydration with the consequent increase of blood viscosity, especially if the athlete cannot, or does not know how to, hydrate themselves well, or there is very abundant fluid loss. It is not unusual for travel to take place soon after the competition. Planes or cars/coaches are vehicles that do not allow for walking for a certain period of time. National flights and bus travels usually last less than two hours, a more than reasonable time to stop and take a rest. Plane trips also have the inconvenience, apart from venous stasis, of being a place where there is a tendency to dehydration due to the low moisture content of the environment41. This aspect should be taken into consideration by athletes during the flight. In regard to height and hydration, it is interesting to note that sports performed at high altitude combine several susceptibility factors, which in themselves have no great influence, but it is not known whether their combination creates an environment favourable to the presence of VTED, hypoxia, dehydration, haemoconcentration, low temperature, tight clothing, reduced mobility, age, previous long trip42. Any suspicious process should alert to an early diagnosis, and any subject with a history should assess their genetic susceptibility (Table 14).
Finally, it should be remembered that there are ergo-genic substances such as anabolic steroids, growth hormones, blood concentrate transfusions, the use of erythropoietin, etc.43 whose aim is to preserve the athlete’s health and guarantee clean competition but which are considered performance enhancing drugs and not expected to be found, but unfortunately they are not infrequent in various areas of physical work, almost always acquired illegally, and causing a kaleidoscope of serious diseases where VTED is highly present. If these conditions affect more predis-posed or associated with certain activities individuals it should be studied, since there seems to be an alarming prevalence of presentation of pulmonary embolism in basketball47, with associated probability individual idiosyncrasies, this event does not occur or does very differently and less conspicuous in professional football48. In the first case, it is possible that the characteristics of sport than those that predispose and facilitate, but select subjects that have them and they are also the result of a lability to present VTE, where the sport environment makes them proof.
On trips of more or less 2* hours, it is necessary to make sure to:
• Walk. When travelling by plane or train, walk for at least five minutes every hour along the aisles of the plane or carriage. When travelling by car or coach, stop every hour and take advantage of the opportunity to walk.
• If the sitting position must be maintained, an attempt should be made to activate the muscles. Bend the knees, move the feet, flex/stretch and rotate the ankles, press the feet progressively against the floor, alternating one, the other, and both at the same time.
• Avoid tight clothing, strong elastics and creases in areas of flexion (Evidence III).
• Drink liquid (non-alcoholic) often and regularly. Hydrate well (Evidence II-3).
On trips of more than 2* hours in addition to the above make sure to:
• Walk > 5 minutes every hour (Evidence II-2).
• Bend the ankles and knees often (Evidence II-2).
• Occasionally raise the legs (above hip level) (Evidence II-2).
If there is a risk of venous thrombosis:
• Use progressive compression stockings (Evidence II-3).
• Take an oral anticoagulant under medical prescription.
• LMWH at preventive doses (Evidence I).
In summary, the recommendations are: be well hydrated, move often, and, if there is any susceptibility, use progressive compression stockings or even LMWH at preventive doses.
Conflict of interests
The authors of this have no conflict of interests. They have no contractual relationship with, nor any personal economic interest in any of the companies whose substances may be mentioned in this text/guide.
* Considering trips of about two hours is an empirical decision based on the maximum duration of flights in Spain or even continental Europe. The majority of trips by coach may exceed this period, although not always reaching the limits of the regulations regarding current driving times and rest periods, EC Regulation No. 561/2006, “Breaks of at least 45 minutes should be taken after four and a half hours at the latest”.
Received 15 June 2015;
accepted: 10 September 2015
* Corresponding author.
E-mail address:drobnic@car.edu, franchek.drobnic@fcbarcelona.cat (F. Drobnic).