Recently, the use of various high-intensity intermittent training (HIIT) that have quick recovery periods are of great importance. Therefore, the purpose of this research was to investigate the acute effect of HIIT training on the serum levels of inflammatory and muscle damage indices in overweight middle-aged men, as well as the kinetics of these markers at 1, 24, and 48 h after HIIT training.
MethodsTwenty-two middle-aged men (40–60 years, BMI 25–30 kg/m2) were divided into two training (n=12) and control (n=10) groups. The HIIT training program consisted of running on treadmill for 30 s with an intensity of 100% maximum aerobic speed (MAV), 30 s active recovery with 50% aerobic speed (4 sets, 4 repetitions and 5 min of rest between each round). Cortisol, IL-6, CRP, ALT, AST, CPK and LDH were evaluated in pre-test, one- hour, 24 and 48 h after HIIT training.
ResultsExcept for the CRP variable, Cortisol, IL-6, CPK, LDH, ALT and AST had a significant increase in one- hour after HIIT compared to the pre-test (P<0.05). Also, IL-6 and CPK variables had a significant increase in 24 h and only CPK had a significant increase in 48 h after HIIT training compared to pre-test (P<0.05).
ConclusionOverall, due to long rest intervals and low activity time during HIIT, inflammatory responses and muscle damage are not severe. The recovery periods of inflammatory and muscle damage markers are faster, so this type of response can be considered an advantage for overweight middle-aged men.
Obesity and overweight are mild inflammatory conditions in which the production of pro-inflammatory cytokines increases by white adipose tissue. Inflammation in obesity is a systematic process and affects a large number of organs,1 although one or more organs may initiate this process. As the intake of calories and fat increases, the activation of inflammatory pathways in cells begins through the perception of nutrients and the delivery of cytokines, and the process of perception of nutrients is done through the recognition of molecular patterns.1 In addition, cytokines are produced in various organs in response to inflammatory stimuli and can act through endocrine, paracrine and autocrine pathways.2
Interleukin 6 (IL-6) also acts as a pro-inflammatory cytokine and an anti-inflammatory myokine which is encoded by the IL6 gene in humans.3,4 IL-6 has anti-inflammatory and anti-inflammatory effects by inducing the release of CRP from the liver. In overweight adults, the levels of inflammatory indicators such as C-reactive protein and interleukins are higher than normal people.5 On the other hand, these inflammatory markers are closely related to the risk factors of cardiovascular diseases and mortality in overweight people. Therefore, fat tissue is known as a metabolically active tissue involved in many physiological, pathological, inflammatory and pro-inflammatory processes of the body, and the hormones secreted from this tissue increase in inflammatory and pro-inflammatory conditions.6
Furthermore, previous studies have suggested that obesity increases cortisol.7 In certain conditions, high cortisol concentrations prevent antibody production and decrease salivary immunity. Besides, obesity and overweight also increase alanine transaminase (ALT), Aspartate transaminase (AST) and gamma-glutamyl transferase (GGT).8 Liver enzymes are associated with obesity, cardiovascular disease (CVD), and CVD mortality. Clinical and population studies have linked liver enzymes with insulin resistance, metabolic syndrome, and type 2 diabetes.9 Not much research has been carried out on the effects of obesity on muscle damage markers including lactate dehydrogenase and creatine kinase. However, some studies have revealed that the amount of lactate transporter in obese people has decreased due to weight loss.10 Exposure to conditions such as intense exercise can also increase inflammation and cause muscle damage. Exercise-induced muscle damage (EIMD) is characterized by symptoms that appear both immediately and up to 24–48 h after the initial exercise. The main consequence of EIMD for the athlete is the loss of skeletal muscle function and pain. It seems that the effect of EMID can be greater in overweight or obese people due to the low level of physical fitness.11,12 High-intensity interval training (HIIT) is a form of interval training, a cardiovascular exercise strategy that alternates short periods of high-intensity anaerobic exercise with periods of lower-intensity recovery. Studies have shown that HIIT training can lead to the control or reduction of inflammatory factors if done over a long period of time, although in the short term and acutely, it may have different effects and increase inflammation.13
However, no clear results have been reported about the acute effects of exercise training, especially HIIT training, on the serum levels of cortisol, CRP, IL-6, liver enzymes, CK and LDH in overweight people. Other research indicates an increase in CRP following a long-term vigorous running session or an anaerobic exercise session in sedentary individuals.14 Some researchers like Costello et al. (2018) and Lira et al. (2017) reported significant increases in CRP, cortisol, and IL-6 following intense exercise training in trained subjects.15,16 In a study, Dorneles et al. (2016) reported that intense intermittent exercise increases LDH and CK. It seems that inflammatory and damaging indicators can increase after intense exercises.17 Studies on muscle damage markers such as CK and LDH have revealed that they increase up to 24 and 48 h after performing sports activities. Therefore, delayed contusion caused by these damaging factors and inflammatory factors is identified 24 to 48 h after exercise.18 Pal et al. (2018) also suggested that interval training makes LDH, CK and liver enzymes increase in 24 and 48 h after training.19 Based on this, the times after HIIT training are very important to check the level of inflammation and muscle damaging markers. Considering that overweight people have low physical fitness, it is important to pay attention to the effects of intense training, especially HIIT training. Therefore, the current study aims to investigate the acute effect of HIIT training on inflammatory and muscle damage indices in overweight middle-aged men, as well as kinetics of these markers in 1, 24 and 48 h after training.
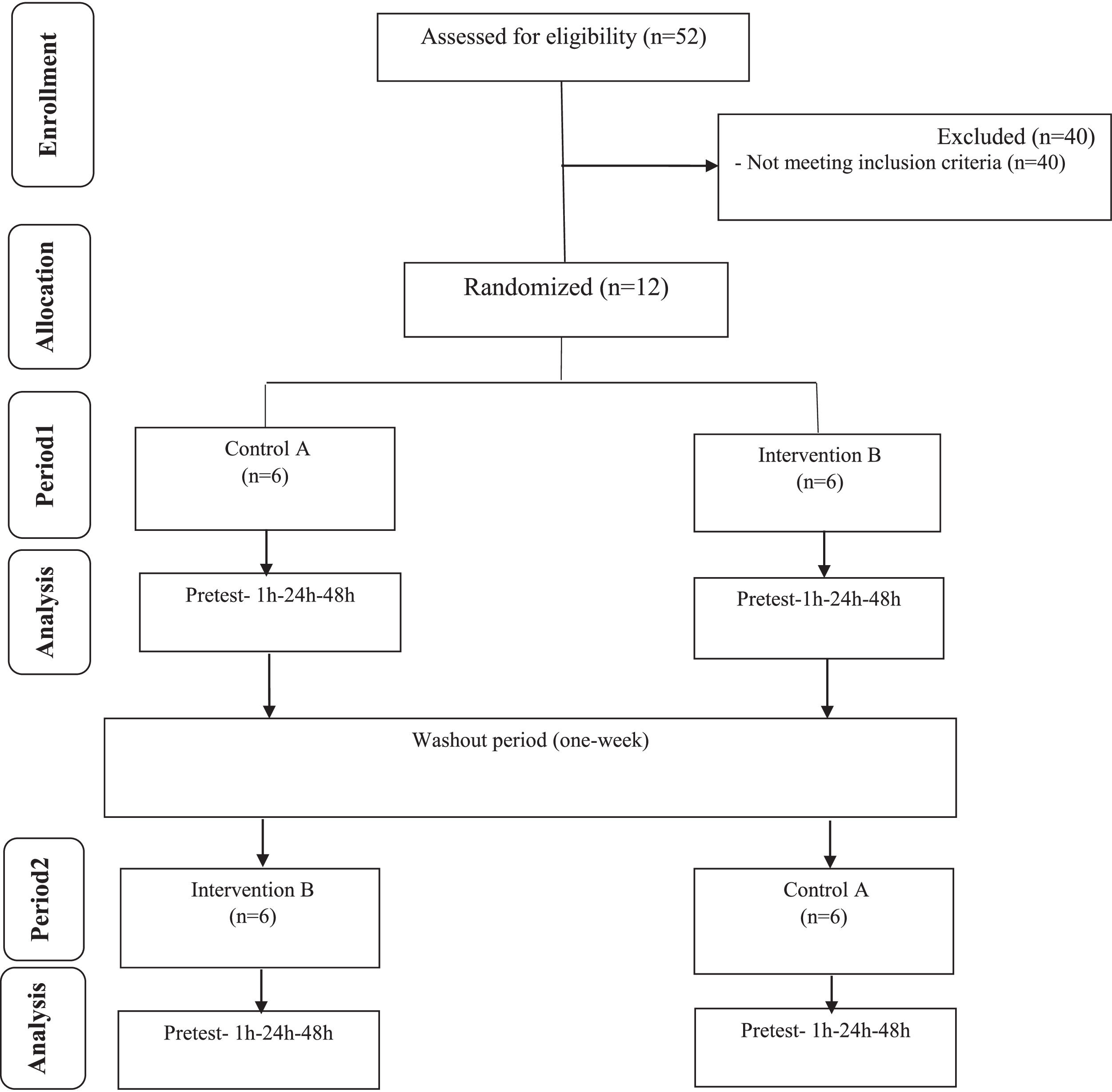
Materials and methodsSubjectsThe present study was performed as an experimental study from January to February 2022, on overweight middle-aged men who referred to the Jahan Sports Gym in Kermanshah, Iran. This research was approved by the ethics committee in biological research of Razi University with the code (IR.RAZI.REC.1400.097). The entry criteria included the age range of 40–60 years, body composition between 25 and 30(kg.m−2) and no movement restrictions. Exclusion criteria included using certain drugs, not using tobacco and alcoholic substances, not having any sports experience in the past one year and not having the history of cardiovascular disease. Obviously, subjects who were harmed or did not meet these conditions or did not participate in practice or measure variables during the research process were excluded from the research process. Also, according to the formula for calculating the sample size and based on the level of significance, statistical power,standard deviation and the main variable of the research, out of the 52 middle-aged men, 22 were selected and divided into two groups of HIIT(n = 12) and control (n = 10) (Fig. 1).20 Research variables were evaluated in the training and control groups at 4 times including the basic phase, one- hour, 24 h and 48 h after training. Randomization was done from a computer-generated sequence, concealed in sequentially numbered, sealed, opaque envelopes, and kept by the gynecological clinic physician. During the implementation of the research protocol, the subjects were asked neither to participate in any additional exercise program and to change their eating style nor to use any special medicine or supplement. Subjects were aware of all aspects of the research and informed consent was obtained from all the subjects.
Anthropometric and physiological measurementsAnthropometric measurements were performed by the researcher on all the subjects based on the reference guide of standard anthropometric measurements.20 The height and weight of the subjects were measured using digital weighing scale and stadiometer (Dena model, DT-102BH). The maximum oxygen consumption was determined using the beep test (multi stage fitness test) which has 21 levels. The speed of the subjects started from 5.8 km/h. In each stage, which lasted approximately one minute, the subject's speed was increased by 0.5 km/h until they could not cover the distance of 20 m determined between the two cones. According to LEVEL and SHUTLEL, aerobic power was calculated using the following formula.21
Moreover, the speed was calculated in the last stage and it was considered as maximal aerobic velocity (MAV) to be used in HIIT training. 1 h, 24 and 48 h after training, venous blood samples were taken to measure biochemical variables from both control and HIIT groups in the stage before exercise. At each stage, 10 ml of blood was taken from the brachial vein in the elbow area. Blood samples were taken in tubes containing EDTA to prevent blood coagulation and transferred to the laboratory in containers containing ice. The blood samples were then centrifuged at a speed of 3200 rpm for 10 min to separate the serum. The serum samples were kept at −80°. Cortisol and Il-6 serum levels were measured using the German Immulite 2000xpi device and the luminescence quantitative method (the German Siemens kit). Then, enzymatic Kits (Delta darman part company kit, Iran) were used to measure AST, ALT, CPK,LDH and CRP(Apetc kit,Spain) .
Familiarization meetingThe subjects visited the training hall before performing the beep test as well as the HIIT training protocol. The purpose of this visit was to acclimate the subjects to the environment of the training hall and the staff, to minimize the learning effect, and also to have them familiarized with the method of performing anthropometric tests, aerobic capacity and HIIT training protocol. They implemented tests and protocols as a pilot.
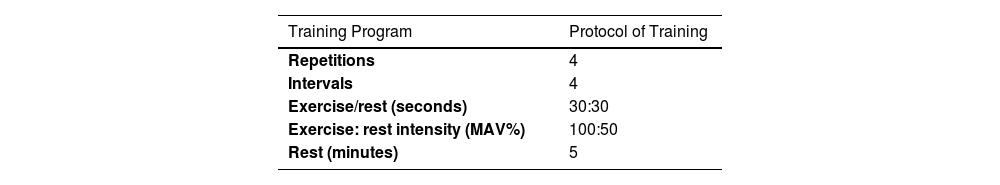
High -Intensity Intermittent Training (HIIT) protocolThe HIIT training program consisted of a warm-up (15 min of standard warm-up) that started with running at a low intensity (50% of MAV) followed by 3 repetitions of 30 s of fast running followed by 30 s of slow running and 5 min of dynamic stretching at the end. The HIIT training program consisted of intermittent running for 30 s at an intensity of 100% maximum aerobic velocity (MAV), 30 s of active recovery at 50% aerobic speed (4 sets, 4 rounds, and 5 min of passive recovery between each round) (Table 2). To control the intensity of the exercises in HIIT training, the subject's heart rate was controlled before the start of the training, during and after the HIIT training in each session by the researcher using Bouerer's heart rate monitor (FT-90, made in Germany).
All training sessions were held at 3:00 to 4:00 (P.M). An emergency physician was also present for primary care while performing functional tests and implementing the training program.
Statistical methodDescriptive statistics methods were applied to describe the mean and standard deviation of the data. Shapiro-Wilk test and two-way ANOVA with repeated measures were used to check the normality of data distribution and to check mean differences, respectively. Independent t-test and Bonferroni's post hoc test were applied for between-groups and within -groups comparison. All analysis was done at a significance level of P < 0.05.
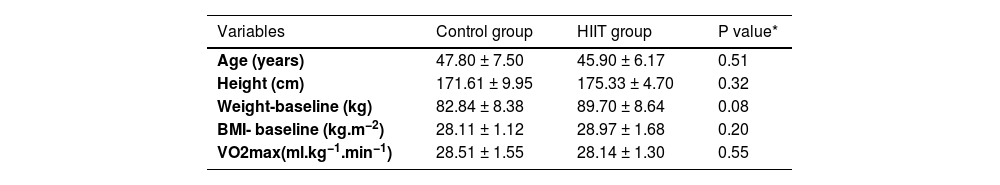
ResultsAs shown in Fig. 1, there were 22 participants in this study. The general characteristics and cardiovascular fitness of patients have been presented in Table 1. As shown, there were no significant variances among the participants in terms of age (P=0.51), height (P=0.32), BMI (P=0.20), weight (P=0.08) and VO2max (0.55) at baseline.
General characteristics of the participants: (Control group n=10, Training group n=12).
| Variables | Control group | HIIT group | P value* |
|---|---|---|---|
| Age (years) | 47.80 ± 7.50 | 45.90 ± 6.17 | 0.51 |
| Height (cm) | 171.61 ± 9.95 | 175.33 ± 4.70 | 0.32 |
| Weight-baseline (kg) | 82.84 ± 8.38 | 89.70 ± 8.64 | 0.08 |
| BMI- baseline (kg.m−2) | 28.11 ± 1.12 | 28.97 ± 1.68 | 0.20 |
| VO2max(ml.kg−1.min−1) | 28.51 ± 1.55 | 28.14 ± 1.30 | 0.55 |
details of HIIT training program: (Control group n=10, Training group n=12).
| Training Program | Protocol of Training |
|---|---|
| Repetitions | 4 |
| Intervals | 4 |
| Exercise/rest (seconds) | 30:30 |
| Exercise: rest intensity (MAV%) | 100:50 |
| Rest (minutes) | 5 |
HIIT: High -Intensity Intermittent and MAV; Maximum Aerobic Velocity.
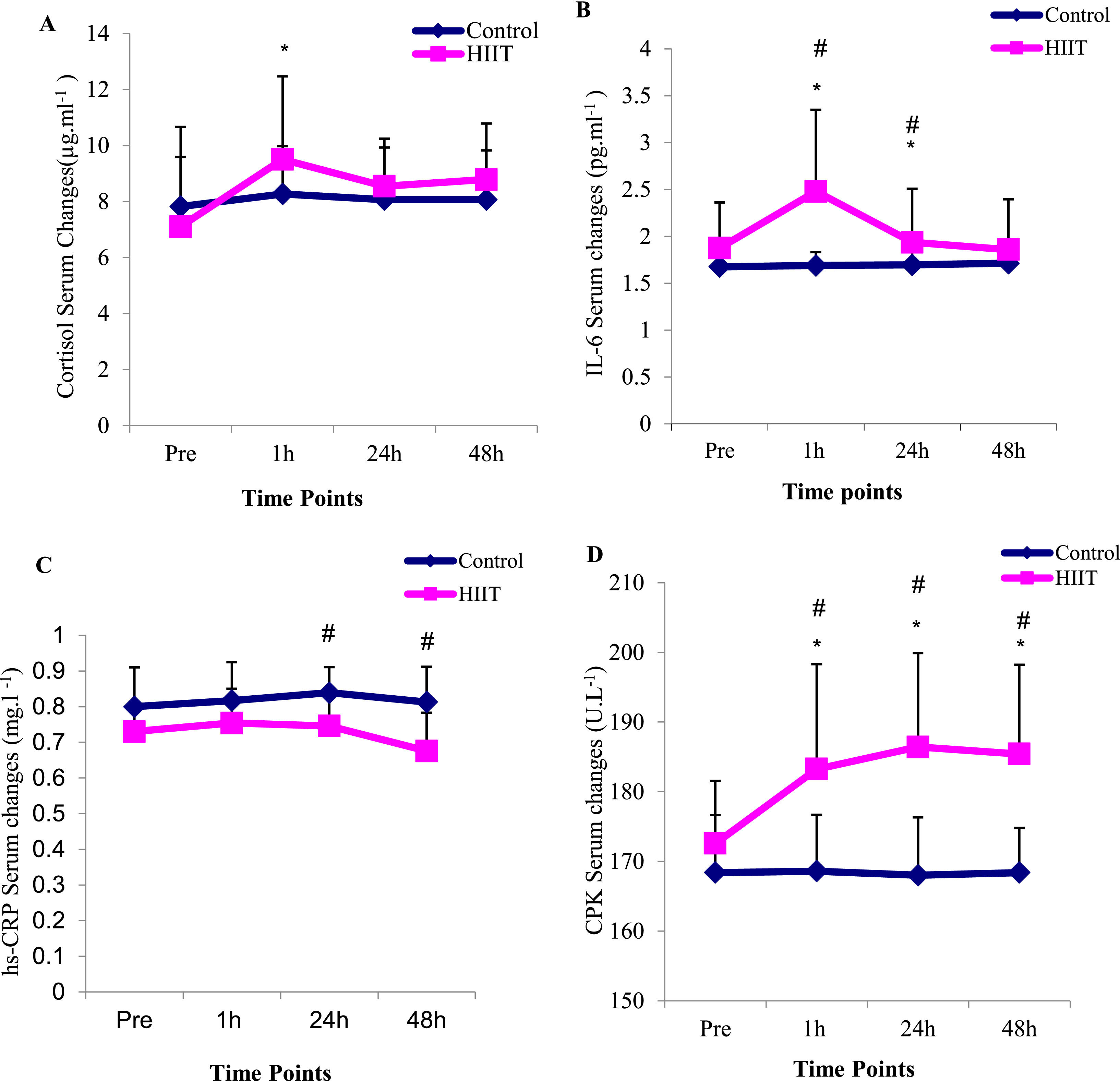
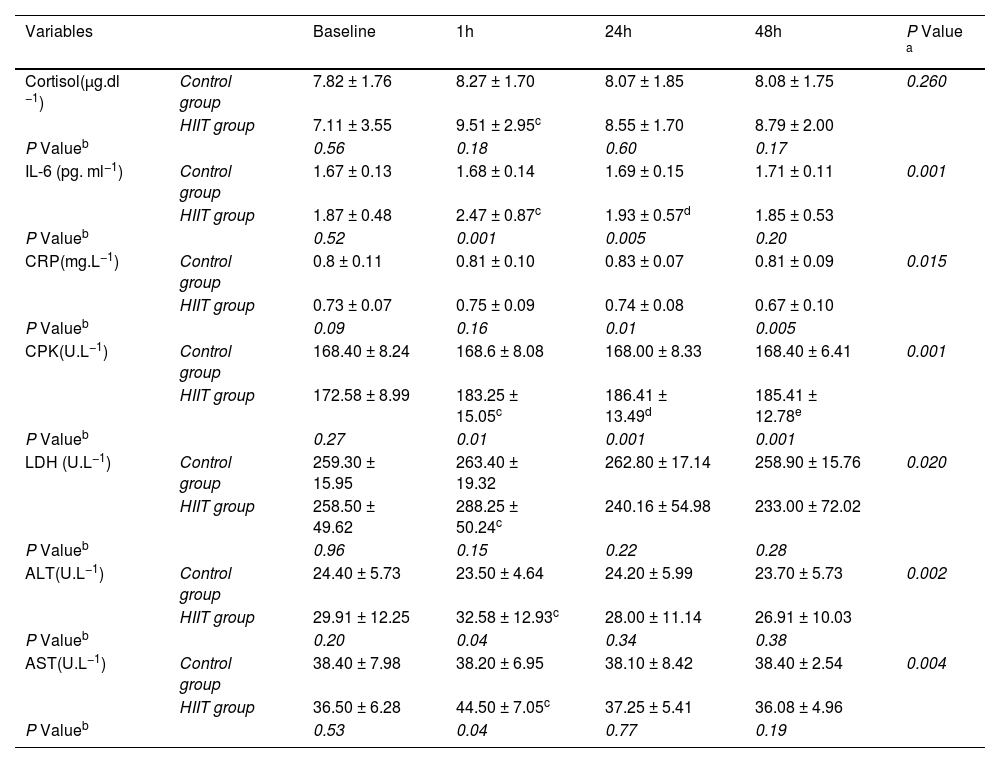
The results of two-way ANOVA test with repeated measurement revealed that the interaction effect of group and time in interleukin 6 variables (P=0.01), hsCRP (P=0.015), CPK (P=0.001), LDH (P=0.02), ALT (P=0.002) and AST (P=0.004) were significant, but in the cortisol variable, the interaction effect was not significant (P= 0.26) (Table 3). Moreover, the results of the Bonferroni post hoc test indicated that there was a significant increase in the variables of cortisol (P=0.001), interleukin 6 (P=0.001), CPK (P=0.005), LDH (P=0.01), ALT (P=0.003) and AST (P=0.001), between one hour after the training and the pre-test, but this difference in hsCRP variable (P=0.2) was not significant (Fig. 2). Also, the results of the Bonferroni post hoc test showed that there is a significant difference between the 24 h with the pre-test in interleukin-6 (P=0.001) and CPK (P=0.001) variables and a significant difference between in the 48 h after training with the pre-test in CPK (P=0.001) variable (Table 3). The results of the independent t-test revealed that in one hour after training there was a significant difference with control group in the variables of interleukin 6 (P=0.01), CPK (P=0.01), ALT (P=0.04) and AST (P=0.04) and in the variables of cortisol (P=0.018), hsCRP (P=0.16) and LDH (P=0.15); these difference were not significant (Fig. 2, Table 3). Besides, the results of the independent t-test showed that there is a significant difference between the training and control groups in the interleukin 6 (P=0.005), hsCRP (P=0.01) and CPK (P=0.001) variables at 24 h after the training (Table 3). The results of this test indicated that there was a difference in hsCRP (P=0.005) and CPK (P=0.001) variables between the two training and control groups 48 h after exercise. In other variables, these differences were not significant (P>0.05) (Table 3).
Comparison of skeletal muscle damage indices, inflammatory markers and cortisol serum levels in two groups of the study (Mean and Standard Deviation).
| Variables | Baseline | 1h | 24h | 48h | P Value a | |
|---|---|---|---|---|---|---|
| Cortisol(µg.dl −1) | Control group | 7.82 ± 1.76 | 8.27 ± 1.70 | 8.07 ± 1.85 | 8.08 ± 1.75 | 0.260 |
| HIIT group | 7.11 ± 3.55 | 9.51 ± 2.95c | 8.55 ± 1.70 | 8.79 ± 2.00 | ||
| P Valueb | 0.56 | 0.18 | 0.60 | 0.17 | ||
| IL-6 (pg. ml−1) | Control group | 1.67 ± 0.13 | 1.68 ± 0.14 | 1.69 ± 0.15 | 1.71 ± 0.11 | 0.001 |
| HIIT group | 1.87 ± 0.48 | 2.47 ± 0.87c | 1.93 ± 0.57d | 1.85 ± 0.53 | ||
| P Valueb | 0.52 | 0.001 | 0.005 | 0.20 | ||
| CRP(mg.L−1) | Control group | 0.8 ± 0.11 | 0.81 ± 0.10 | 0.83 ± 0.07 | 0.81 ± 0.09 | 0.015 |
| HIIT group | 0.73 ± 0.07 | 0.75 ± 0.09 | 0.74 ± 0.08 | 0.67 ± 0.10 | ||
| P Valueb | 0.09 | 0.16 | 0.01 | 0.005 | ||
| CPK(U.L−1) | Control group | 168.40 ± 8.24 | 168.6 ± 8.08 | 168.00 ± 8.33 | 168.40 ± 6.41 | 0.001 |
| HIIT group | 172.58 ± 8.99 | 183.25 ± 15.05c | 186.41 ± 13.49d | 185.41 ± 12.78e | ||
| P Valueb | 0.27 | 0.01 | 0.001 | 0.001 | ||
| LDH (U.L−1) | Control group | 259.30 ± 15.95 | 263.40 ± 19.32 | 262.80 ± 17.14 | 258.90 ± 15.76 | 0.020 |
| HIIT group | 258.50 ± 49.62 | 288.25 ± 50.24c | 240.16 ± 54.98 | 233.00 ± 72.02 | ||
| P Valueb | 0.96 | 0.15 | 0.22 | 0.28 | ||
| ALT(U.L−1) | Control group | 24.40 ± 5.73 | 23.50 ± 4.64 | 24.20 ± 5.99 | 23.70 ± 5.73 | 0.002 |
| HIIT group | 29.91 ± 12.25 | 32.58 ± 12.93c | 28.00 ± 11.14 | 26.91 ± 10.03 | ||
| P Valueb | 0.20 | 0.04 | 0.34 | 0.38 | ||
| AST(U.L−1) | Control group | 38.40 ± 7.98 | 38.20 ± 6.95 | 38.10 ± 8.42 | 38.40 ± 2.54 | 0.004 |
| HIIT group | 36.50 ± 6.28 | 44.50 ± 7.05c | 37.25 ± 5.41 | 36.08 ± 4.96 | ||
| P Valueb | 0.53 | 0.04 | 0.77 | 0.19 |
Abbreviations: h, Hour; IL-6, Interleukin-6; hs-CRP, High- sensitivity C -reactive protein; CPK, Creatine phosphokinase; LDH, Lactate dehydrogenase; ALT, Alanine transaminase; AST,Aspartate aminotransferase.
P values superscript with “a” is calculated using repeated measures ANOVA test for comparing interaction of times × groups; P values superscript with “b” is calculated using independent t-test for comparing between groups at each time point.
This study mainly aims to investigate the acute effects of HIIT training (intensity 100% of MAV, 6 sets and 4 rounds) on the serum levels of inflammatory (cortisol, CRP, IL-6) and muscle damage indices (CK and LDH and liver enzymes) in over weight middle-aged men (40–60 years old, BMI 25–30 kg m−2) and kinetic of these markers after training. The findings revealed that HIIT training led to a significant change in all inflammatory and muscle damage variables in the training group in one hour after the training compared to the pre-test. Also, the results of the research showed that HIIT training could not cause a significant change in cortisol, CRP, and LDH variables in one hour after training compared to the control group, although these differences were significant in IL-6, CPK, ALT, and AST variables. Furthermore, the results showed that at 24 h and 48 h after training, no difference was observed between the training and control groups in the variables of cortisol, LDH, ALT and AST.
The results of the present study indicated that IL-6 serum levels in the HIIT training group increased significantly in the one-hour and 24 h phases after the HIIT training compared to the pre-test. The increase of IL-6 in one- hour after the training compared to the pre-test phase was almost 32%. Besides, a significant difference was observed between the two groups in the level of IL-6 at one- hour and 24 h after the training. According to the results of this research, the serum levels of CRP in the HIIT training group one- hour after the training had a small increase, although it was not significant. The increase of CRP in one- hour after training compared to the pre-test phase was almost 6%. Also, there was a significant decrease in the 48 h after training compared to the one-hour (P=0.002) and 24 h (P=0.001). However, its value was significantly higher than the control group at the 24 h and 48 h. These findings are in accordance with other research. Zwetsloot et al. (2014) reported a small increase in IL6 levels after intense interval training in active young men13; Arent et al. (2010) reported a 2–2.5-fold increase in IL-6 concentration after performing interval running and cycling in athletes.22 However, contrary to these findings, Shanely et al. (2014) indicated a 4–40-fold increase in IL6 concentration up to 1.5 h after continuous and long-term aerobic activity.23 It seems that HIIT training appears to induce a less intense inflammatory response compared to long-term continuous aerobic exercise. These differences are likely due to the relative time spent training between HIIT and prolonged physical activity.13
Considering the role of Il-6 in the inhibition of pro-inflammatory cytokines (IL-1β and TNF-α) as well as the activation of anti-inflammatory cytokines (IL-1ra, IL-10) and glucose metabolism, several mechanisms are responsible for increasing the serum levels of Il-6 in during and after the activity. It has been suggested that, among other things, it is possible to mention the increase in the production of free radicals and the activation of the NF-KB signaling pathway and the increase in heat shock proteins (HSPs) during HIIT training.24,25
Furthermore, Sarkar et al. (2021) showed that temporary hypoxia caused by HIIT training is a strong stimulus to increase interleukin-6 levels.26 Fesicher et al. (2005) also revealed that the duration, intensity and recovery time are the determining factors in the acute phase response (APR) to the kinetics of IL-6. More than 50% of the changes in IL-6 are related to the duration of exercise, and recovery time can also affect the inflammatory response.24
Brown et al. (2018) suggested that even 48 h after exercise training, IL6 levels are higher than resting levels. Also, these researchers state that increasing IL-6 levels after exercise training causes muscle pain, reduced glycogen availability, and changes in calcium homeostasis caused by activation of mitogen-activated protein kinase (MAPK) and increased stress hormones.24,26,27 Previous studies have shown that HIIT produces a small inflammatory cytokine response; However, long-term continuous aerobic exercise produces a much larger inflammatory cytokine response than short-term.22,25,28,29
Knab et al. (2013) reported that long-term, high-intensity interval swimming (2 h, 1:1 and 1:2 swim-to-rest ratio) produced a small increase in IL-6 suggesting that rest intervals have a profound attenuating effect on the inflammatory response to high-intensity physical activity30; the increase of IL-6 can be effective in increasing the amount of CRP.31,32 Kasapis et al. (2005) showed that hypoxia caused by HIIT training is a stimulus to increase CRP levels. In this regard, Baygutalp et al. (2021) reported that the more physical activity leads to hypoxia, the higher the level of CRP. Contrary to these findings and in line with our findings, Markovitch et al. (2008) did not observe any changes in CRP levels after moderate aerobic exercise in middle-aged men.33 The equivocal findings of these studies can be attributed to several methodological factors, although the only obvious differences lie in the exercise stimulus and participants' body fat percentage. Lin et al. (2010) suggested that the increase of CRP is highly related to the intensity of the exercise and the percentage of body fat mass. Adipose tissue releases several adipokines that mediate inflammation and promote hepatic synthesis of CRP releasing into the circulation. It seems that the greater the fat tissue size of the subjects, more CRP increases in one-session intense training. According to these factors, it seems that despite the high -intensity of the training and the overweight of the subjects in the present study (body composition between 25 and 30), CRP levels did not increase between repetitions and sets in HIIT training which is because of the rest.
The findings fron the present study also showed that the serum levels of cortisol in the training group increased significantly in the one-hour post-exercise phase compared to the pre-test. The amounts of cortisol increase in one-hour after training compared to the pre-test was almost 63%. In other phases, its value decreased and approached the pre-training phase. The results of the independent t test revealed that there is no significant difference between the two groups in the amount of cortisol serum levels in all stages. The findings are supported by Dote-Montero et al. (2021) in which they showed that the sharp increase in cortisol serum levels after HIIT training can be the result of a decrease in plasma volume, liver clearance, and the rate of tissue destruction.34 Another reason to explain the increased cortisol response under hyperoxia is related to the increased formation of reactive oxygen species or free radicals. Free radicals have been shown to reduce or even abolish the negative feedback regulation of glucocorticoids. This process, in turn, leads to an increase in plasma cortisol levels.35 Cortisol concentrations increase linearly with exercise intensity,36 so it takes longer for cortisol to return to baseline levels after high-intensity intermittent exercise such as HIIT. This process could explain why cortisol levels remained high 60 min after a HIIT session,37 which is in line with the results of our research.
Based on the results of the present research, the values of ALT and AST in the HIIT training group increased significantly in one-hour after training compared to the pre-test. The increase of ALT and AST in one- hour after training compared to the pre-test was approximately 10 and 24%, respectively. There was a significant difference between the two groups in the levels of ALT and AST at the one-hour post exercise. However, there was no difference between the two groups at 24 h and 48 h post exercise.
The findings are supported by Turgut et al. (2017) in which they revealed that after a session of swimming training, ALT and AST values increased in obese women.38 Ramos et al. (2013) also reported a significant increase in liver enzymes as a result of an intense training session39 which indicates that muscle damage has occurred. Therefore, it seems that in the current research, part of the increase in the serum levels of liver enzymes (ALT and AST) is related to the increase in muscle damage caused by high-intensity interval training. It increases the level of ALT and AST enzymes, a trend that is less seen in long-term training.40 Intense physical activity increases blood flow in working skeletal muscles and decreases blood flow in the liver and portal vein. Then, decreased blood flow to the liver causes liver damage. It seems that the decrease in blood flow in the liver causes hypoxia of the liver cells and finally causes necrosis. This process suggests that intense physical activity may cause hepatic necrosis or ischemic reperfusion.41 High-intensity physical activity appears to cause changes in liver function and the number of damaged liver parenchymal cells. Ischemia-perfusion increases inflammation and can increase the production of free radicals.41,42
CPK and LDH are well recognized as potentially important markers of muscle damage.43,44 The results of the research indicated an increase in CPK levels in the training group at one-hour, 24 h and 48 h after training compared to the pre-test. There was also a difference between the two groups in the level of CPK at one- hour, 24 h and 48 h after training. These increases in CPK and LDH serum levels in one -hour after training were approximately 9 and 13%, respectively. The amount of LDH also increased significantly in the training group in the one-hour post-exercise compared to the pre-test stage. There was no significant difference between the two groups in the amount of LDH at different post exercise times.
The findings are in line with Callegari et al. and Tesema et al. (2019) in which they observed a 100–200% increase in CPK and LDH values after acute / moderate and chronic / high-intensity exercise training, after 45 and 30 min after training, respectively. This increase lasted up to 24 h after training. These researchers stated that all muscle injuries occur due to the shortening stretch cycle during muscle activity in short periods of high-intensity training.43,44 The creation of stress-stretch characteristics in skeletal muscle fibers leads to severe micro-damages in the sarcolemma and ultimately causes the leakage of CK and LDH in the blood stream as an indicator of damage.12,35 In addition, there is a significant correlation between changes in cortisol concentration and blood lactate accumulation. It has been shown that cortisol responses are greater in people who have a greater increase in blood lactate.35 In the present study, cortisol and LDH increased significantly one -hour after HIIT training and then returned to baseline values.
The results of the study by Cipryan et al. (2017) revealed that all three HIIT protocols (15 s, 30 s or 60 s with a work-to-rest ratio of 1:1) increase muscle damage markers in blood circulation.45 In addition, Ashtari et al. (2017) showed a significant increase in CK activity in both trained and untrained groups, immediately and one- hour after intense resistance training compared to the pre-training.46 Also, the amount of LDH increased significantly immediately after training in both groups and decreased one- hour after training compared to immediately after training phase.
Despite the use of HIIT training (100% MAV,4 repetitions and 4 sets) in overweight men in the current study, which is considered as one of the strengths, the responses created in the research variables cannot be compared due to the different intensities in high -intensity interval training. The lack of comparison of the research parameters in gender and the small sample size due to the unavailability of the samples were the limitations of the research. It is recommended next research be conducted with a larger sample size and examine the chronic effects of this type of high-intensity intermittent.47 In conclusion, these results show that despite the high- intensity of training in this type of training due to long rest intervals and low activity time, inflammatory responses and muscle damage are not severe. The recovery periods of inflammatory and muscle damage markers are faster, so this type of response can be considered an advantage for overweight middle-aged men.
CRediT authorship contribution statementBehnam Rohnejad: Conceptualization, Formal analysis, Writing – review & editing. Amirabbas Monazzami: Conceptualization, Writing – original draft, Formal analysis, Data curation, Writing – review & editing.