Despite the implementation of specific exercises to reduce hamstrings strain injuries (HSI) risk, the incidence has remained unchanged over the past 30 years. Therefore, the purpose of the study was to analyze hamstrings muscle activation induced by a novel Flywheel Russian belt Deadlift (FRD) exercise, together with individual muscle-, region- and limb-specific differences.
MethodsThe activation of hamstring muscles before and immediately after a 10 × 10 FRD training session was assessed by the T2 shift technique through functional magnetic resonance imaging, in one international-level filed hockey male player. The individual use of the biceps femoris long head (BFLH) and short head (BFSH), semitendinosus (ST), and semimembranosus (SM) were analyzed, together with the region-specific activation for each muscle.
ResultsT2 values significantly increased immediately after exercise in all regions of the hamstring muscles in both dominant and non-dominant lower limb. However, the SM muscle showed a lesser activation, compared to BFLH, BFSH and ST muscles [F(3,106) = 9.557, p < 0.001]. Overall, the most activated muscle by the FRD training session was the biceps femoris (short head +13.8 %, long head, +12.7 %), followed by the ST (+11.3 %), and lastly the SM (+6.9 %).
ConclusionThe novel FRD exercise triggers a homogeneous and consistent activation of hamstring muscles. Particularly, the lateral positioning muscles (i.e., BFLH and BFSH) were more activated than the medial positioning muscles (i.e., ST and SM). Therefore, the FRD exercise could enhance training programs to strengthen and activate the hamstring muscles, and specifically, the BFLH, in order to reduce HSI risk.
Hamstring strains injuries (HSI) are among the most common musculotendinous injuries in team sports in both males and females, by increasing the burden and financial cost, and decreasing the performance and quality of life of athletes.1 Predominantly, repeated sprints are one of the most frequently high-intensity actions in team sports, leading to a higher HSI risk.2 During sprinting, the horizontal ground reaction force is predicted by the hamstrings electromyographic activity and eccentric torque during the late swing phase of the running gait cycle.3 The late swing phase is a key point of the sprint, involving two simultaneous and damaging events. First, the maximum length of the hamstrings muscle-tendon unit is reached. Concurrently, the hamstrings undergo an active, eccentric lengthening contraction.4 Overall, repeated hamstrings eccentric contractions during repeated late swing phases of the running gait cycle are the main mechanism contributing to HSI.5 In particular, the biceps femoris long head (BFLH) has been found to be most important hamstring muscle to decelerate the shaft during the late swing phase.6,7 The reason is that the lower limbs positioning during the late swing phase causes a greater BFLH stretching, and moment arm, compared to the semitendinosus (ST) or semimembranosus (SM) muscles,4 and explains why biceps femoris strains are the most common HSI in sprint-based team sports in both males and female athletes.2
Focusing on prepare the hamstrings for the repeated eccentric contraction-derived muscle strains during repeated late swing phases of the running gait cycle is therefore essential to both reduce the HSI risk and improve performance.8,9 Although resistance-based training is the most popular method, different approaches to hamstrings training have been explored, including eccentric training, inertial training, knee-dominant or hip-dominant exercises. The Nordic hamstring exercise has become one of the most widely used exercises to train the hamstrings since it provides a large eccentric workload. However, magnetic resonance imaging (MRI)-based studies have demonstrated that this knee-dominant exercise causes great activations of the ST and biceps femoris short head (BFSH).10-13 On the other hand, flywheel inertial training is a recognized training method to reach eccentric overload (EO),14 allowing to prepare the eccentric phase of a movement. The most commonly used flywheel exercise to train the hamstrings is the flywheel prone leg curl which, similar to the Nordic hamstring exercise, generates a high activation of the ST and BFSH, but not BFLH.10,11 Contrary to the above-mentioned knee-dominant exercises, hip-dominant exercises, such as the Russian belt deadlift or the unilateral hip extension conic-pulley, seem to provide the most stimulation to the BFLH, compared to the other hamstrings muscles, although modest T2 shift were shown (i.e., 7 % and 6 % respectively).10 The reason is that hip-dominant exercises activate the lateral hamstrings more than the medial hamstrings, representing a 4-times greater activation of the BFLH compared to the ST.15 Specifically, the product of the physiological cross-sectional area and the hip moment arm is higher for the BFLH compared to the ST muscle,16 so during hip-dominant movements, there is a strong participation of the BFLH.15,17
Therefore, the actions that shifts the center of gravity forward of the transverse axis of the hip joints (e.g., forward bending of the trunk with hip flexion) cause a significant contraction of the lateral hamstring muscles, particularly the BFLH.18,19 In short, depending on the selected exercise and technique, there is a selective recruitment of the hamstrings, with the BFLH being preferentially and eccentrically activated when the hip flexes and/or the knee extends in order to decelerate the movement.16
Despite the above-mentioned approaches for hamstrings training, the injury incidence has remained unchanged during the last 30 years.1 Considering that (i) loading exercises during extensive lengthening reduce the time to return to play, and (ii) active trunk stabilization during exercise decreases reinjury rates,20 it is reasonable that hamstrings training, particularly BFLH, should be based on a hip-dominant exercise with special emphasis on working active eccentric lengthening contractions conducted with high loads and long musculotendon lengths.8,9 Consequently, it is proposed to combine the Russian belt deadlift with flywheel training to build a new Flywheel Russian belt Deadlift (FRD) exercise. Given that the selection of exercises targeting specific muscles in injury prevention or rehabilitation programs can be conducted by muscle functional MRI (mfMRI),17 the purpose of the study was to determine exercise-induced hamstring muscles activation (T2 shift) immediately after a FRD training session, together with individual muscle-, region- and limb-specific differences.
Materials and methodsExperimental approachOne international-level filed hockey male player (age = 22.7 years, mass = 67.0 kg, height = 1.72 cm) voluntarily accepted to participate in the case study, in order to assess hamstring muscles activation before and immediately after the FRD training session. The volunteer gave their written consent that received information about the purpose of the study and associated risks. The experiment was conducted in accordance with the code of ethics of the World Medical Association (Declaration of Helsinki) and was approved by the Ethics Committee for Clinical Research of the Catalan Sports Council (Generalitat de Catalunya) (037/CEICGC/2021).
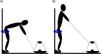
Flywheel Russian belt Deadlift exerciseThe training session started with a standardized warm-up, consisting of dynamic joint mobilization and 2 sets of 8 repetitions of the FRD exercise under progressively increasing and submaximal effort. Then, the FRD training session consisted of 10 sets of 10 repetitions conducted on a conical flywheel device Proinertial pulley pro C2 (Inertial systems S.L., Barcelona, Spain), with a 3-minute rest period between sets. The inertia load was set at 0.124 kg·m², as greater moments of inertia lead to greater EO.21 Given that the first repetitions of inertial training are used to increase the speed of the flywheel and are considered not to be effective repetitions, the first three repetitions of each set were used to “increase momentum” and were excluded from the data analysis.22 The standardized starting position during the novel FRD exercise was with the feet on a 30-degree sloping wedge, the lower limbs and the body weight held by a Russian belt attached to the wall, self-selected knee flexion between 0 and 10°, hip flexion down to 90°, and abdominal bracing together with scapular retraction (Fig. 1A). Then, the exercise started conducting a hip extension to 0°, i.e., concentric phase, (Fig. 1B), and back to hip flexion to the bottom of the range of motion, i.e., eccentric phase. The volunteer was verbally encouraged to perform the concentric phase as fast as possible, and to brake as hard as possible during the last part of the eccentric phase in order to increase EO.23
Image acquisition and processingThe activation of hamstring muscles before and immediately after the FRD training session was assessed by the T2 shift technique through mfMRI.24 To minimize the effects of fluid shifts caused by walking, the individual remained recumbent for a minimum of 10 min before basal condition image acquisition, while immediately after the exercise the individual was wheelchair-assisted between the exercise room and the MRI scanner.24,25 Then, the individual was placed on the magnetic resonance scan Vantage Galan 3T (Cannon Medical Systems, Tochigi, Japan), in supine position with his thighs covered with two overlapped 16-channel Atlas SPEEDER body coils. A custom-made foot-restraint device was used to maximize the repeatability of limb placement in the MRI scanner. T2-weighted imaging was acquired with the following parameters: TE 20, 60, 100 and 140 ms; TR 3200 ms; in-plane resolution 0.25 × 0.25 mm; slice thickness 3 mm; gap 18 mm. Total acquisition time: 6 min. Pre-exercise acquisitions also included axial fat-supressed proton density-weighted imaging with TE 33 ms; TR 2954 ms; in-plane resolution 0.49 × 0.49 mm; slice thickness 3 mm; gap 18 mm. The imaging protocol was conducted in two blocks to cover the extension of hamstring muscles from the distal margin of the ischial tuberosity to the tibial plateau.
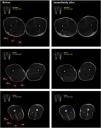
Subsequently, a parametric image was generated from the T2 mapping sequence using parametric MRI (pMRI) software v.1.3.3-b (Philadelphia, PA, USA). The MRI data were then evaluated for T2 relaxation time (T2 value) of the hamstring muscles. A circular region of interest (ROI) was defined for the BFLH, BFSH, ST, and SM muscles in each of the T2 mapping images where these muscles were visible. Intramuscular vascular structures, connective tissue and the boundaries of the muscles were avoided as the T2 values could be affected. ROIs of similar size and anatomical location were placed in the subsequent image sets to ensure positioning identical to that in the basal analysis (Fig. 2).11 A multi-echo 2DFSE T2-weighted was applied to measure the mean T2 value. Images taken at different TEs were fit to a monoexponential time curve to extract the T2 values.26 Region-specific muscle activation for each muscle were computed as the mean T2 absolute value of the different ROIs containing of the areas at 0–30 % (proximal), 30–70 % (middle) and 70–100 % (distal) of thigh length, from the lower border of the ischial tuberosity (0 %) to the upper border of the tibial plateau (100 %).26 T2 shift was finally calculated by subtracting T2 baseline values from T2 post-exercise values and expressed as a percentage of the difference.27 Two independent researchers conducted the MRI scanning and the T2 shift analysis.
Selected magnetic resonance images acquired before and immediately after the Flywheel Russian belt deadlift training session, depicting regions of interest (ROIs). 1, m. biceps femoris short head (BFSH); 2, m. biceps femoris long head (BFLH); 3, m. semitendinosus (ST); 4, m. semimembranosus (SM).
A four-way mixed model [time (pre, post) x muscle (BFSH, BFLH, ST, SM) x limb (dominant, non-dominant) x region (proximal, middle, distal)] was conducted to determine exercise-induced hamstring muscles activation (T2 values). Subsequently, a three-way mixed model [muscle (BFSH, BFLH, ST, SM) x limb (dominant, non-dominant) x region (proximal, middle, distal)] was used to determine differences in hamstring muscles T2 shift immediately after the FRD exercise. Bonferroni's corrected post hoc analysis was conducted if the model showed statistically significant main effects or interaction effects. Data are presented as mean ± standard deviation (SD) and the level of significance was set at p < 0.05. Statistical analysis was performed with SPSS v.27.0.1.0 (SPSS Statistics, IBM Corp., Armonk, NY, USA).
ResultsRepresentative T2-weighted magnetic resonance images before and immediately after the 10 × 10 FRD training session are presented in Fig. 3. A significant main effect for time were observed for the T2 values [F(1106) = 51,838.462, p < 0.001]. T2 values substantially increased from the basal condition to immediately after exercise in all regions of all the hamstring muscles in both dominant and non-dominant lower limb (Table 1).
Representative T2-weighted magnetic resonance images of the proximal region (25 % of thigh length), middle region (50 % of thigh length) and distal region (75 % of the tight length) before and immediately after the Flywheel Russian belt deadlift training session. BFSH, m. biceps femoris short head; BFLH, m. biceps femoris long head; ST, m. semitendinosus; SM, m. semimembranosus.
Region-specific T2 values, mean difference (MD) and percentage of the difference (%Δ) of hamstring muscles before and immediately after the Flywheel Russian belt deadlift training session.
(∗) indicates significant post-exercise change from baseline T2 value. %Δ, percentage of delta change; BFSH, m. biceps femoris short head; BFLH, m. biceps femoris long head; MD, mean difference; ST, m. semitendinosus; SM, m. semimembranosus.
A significant main effect for muscle were observed for T2 shift [F(3106) = 9.557, p < 0.001]. T2 shift of the SM was significantly smaller after the exercise in both dominant and non-dominant limb, compared to BFLH, BFSH and ST (Fig. 4). There were no between-limbs [F(1106) = 0.403, p = 0.527] nor between-regions [F(2106) = 1.564, p = 0.214] differences in hamstring muscles T2 shift. There were also no significant muscle-limb [F(3106) = 0.096, p = 0.962], muscle-region [F(5106) = 0.670, p = 0.647], limb-region [F(2106) = 1.152, p = 0.320], nor muscle-limb-region [F(5106) = 0.766, p = 0.576] interactions. Therefore, the most activated muscles by the FRD training session, marked by T2 shift, were the biceps femoris (short head +13.8 %, long head, +12.7 %), followed by the ST (+11.3 %), and lastly the SM (+6.9 %).
Mean and standard deviation of the change in the transverse relaxation time (%T2 shift) of the 30 % (proximal), 50 % (middle) and 70 % (distal) regions of thigh length in m. biceps femoris short head (BFSH), m biceps femoris long head (BFLH), m. semitendinosus (ST), and m. semimembranosus (SM), immediately after the Flywheel Russian belt deadlift training session. All values are given as a percentage of the pre-value. (*) indicates significant T2 shift and (#) indicates substantial differences in SM muscle, compared to BFSH, BFLH and ST muscles. D, dominant limb; ND, non-dominant limb.
Individual muscle-, region- and limb-specific differences in hamstring muscles activation (T2 shift) were assessed immediately after a novel FRD exercise. The findings of the study highlight that all hamstring muscles were activated during the FRD training session. Particularly, the biceps femoris (i.e., BFLH and BFSH) was the most activated hamstring muscle, followed by the ST, while the SM was activated to a lesser extent. Finally, there were no between-region nor between-limb differences in hamstring muscles activation after the FRD training session.
The transverse relaxation time (T2 value) of hydrogen protons in skeletal muscle can be quantified by mfMRI. Changes in the T2 value between before and after a single exercise (T2 shift) have been described as a highly reliable and non-invasive indicator of muscle activation during exercise.24 The proposed FRD exercise significantly increased the T2 shift of all the hamstring muscles, ranging from 6.9 % for the SM to 13.2 % for the biceps femoris. To our knowledge, no previous MRI-based study has reported activations of all hamstring muscles after a single exercise. In fact, a comparative study of four frequently used hamstring exercises (i.e., Nordic hamstring exercise, flywheel leg curl, Russian belt deadlift and hamstrings kick coney-pulley) found that neither exercise was able to significantly target all hamstring muscles.10 As examples, a 4 × 8 flywheel leg curl training session with a moment of inertia of 0.072 kg·m² or body-weighted Nordic hamstring exercise provided large T2 shifts of the ST and BFSH,10 and are recommended when the goal is to target knee-dominant hamstring muscles.11 Conversely, hip-dominant exercises such as the Russian belt deadlift or the hamstrings kick conic-pulley were effective in triggering the activation of the BFLH (7 % and 6 %, respectively), although the remaining hamstring muscles were not activated.10 Using a Russian belt to hold the body weight and bend the trunk forward together with small knee flexion (between 0° and 10°), the FRD exercise allowed to achieve a more stretched positioning of the hamstrings. Therefore, the stretching of the hamstrings, together with the large EO workload provided by the flywheel inertial device, leads to focusing the exercise on working active eccentric lengthening contractions conducted with high loads and long musculotendon lengths,8,9 which are HSI risk factors during the late swing phase of the running gait cycle.5
The biomechanical response of each hamstring muscle is heterogeneous, given that they display a variety of force-length properties and angle-specific joint torques.16 The hereby proposed FRD exercise elicited the greatest activation of the biceps femoris. Specifically, the BFLH is more sensitive to hip extension than knee flexion because the peak in the length-tension relationship of the BFLH occurs with the hip at 90° and the knee at 0°, the point at which the greatest BFLH function is produced.28 Furthermore, lower-limb positioning near to hip flexion (i.e., 90°) with the knee extended (i.e., 0°) selectively activates the BFLH.29 Thus, during hip-dominant exercises (e.g., forward bending of the trunk with hip flexion), the hamstrings are responsible for driving the movement, with a strong participation of the BFLH.15 Conversely, the BFLH involvement is restricted in knee-dominant exercises since hip movement is locked, and explains the large ST and BFSH activations during knee-dominant exercises.10–13,26,30,31 Region-specific (i.e., proximal, middle, distal) differences in hamstring muscles activity during hamstring exercises has been investigated before. The hamstrings kick conic-pulley has shown greater BFLH proximal and middle activation compared to the other hamstring muscles, albeit to a small extent.11 In contrast, the Nordic hamstring exercise and the flywheel leg curl have shown the same ST and BFSH activation in all muscle regions, while the Russian belt deadlift seemed to be the unique exercise capable of activate the SM across muscle regions.11 Given the Russian belt use during the proposed FRD exercise, SM muscle activation was also effective in all regions of the thigh. Similarly, the FRD exercise resulted in no region-specific differences in BFLH, BFSH and ST activation. Overall, the FRD exercise appears to provide a significant and homogenous (i.e., all muscle regions) hamstring muscles activation.
The present study proposes the combination of the Russian belt deadlift with flywheel training to build a novel Flywheel Russian belt Deadlift exercise which significantly increases the activation of the hamstrings, with special focus on the BFLH due to the injury incidence in sprint-based team sports. It has been found that the designed FRD exercise is a promising training method to produce significant active eccentric lengthening contractions conducted with high loads and long musculotendon lengths in the biceps femoris, followed by the ST, and lastly the SM. However, the study presents limitations to consider when interpreting the results. First, the design of the study provides limited sample power, and therefore generalizing the findings should be cautious. In addition, post-exercise muscle recovery indicators (i.e., force-generating capacity, perceived soreness) were not monitored, thus the short-term effect of the FRD exercise on hamstrings neuromuscular capacities is still unknown. Given the limitations of the study, future studies increasing the sample size to achieve greater statistical power, and monitoring hamstrings muscle recovery after a FRD training session are warranted. In addition, studies on the effects of a long-term FRD exercise-based training program on HSI incidence and muscular performance would be meaningful.
ConclusionThe FRD exercise is able to trigger a homogeneous and consistent activation of all the hamstring muscles (i.e., BFLH, BFSH, ST and SM) throughout the hamstring regions and both dominant and non-dominant limbs. More specifically, the BFLH and BFSH were the most activated hamstring muscles, followed by the ST, and lastly the SM. Therefore, training programs in order to strengthen and activate the hamstring muscles, and particularly the BFLH, could be improved by the inclusion of the FRD exercise.
CRediT authorship contribution statementPedro Luis Cosio: Conceptualization, Methodology, Investigation, Data curation, Formal analysis, Writing – original draft, Writing – review & editing. Lia Moreno-Simonet: Conceptualization, Investigation, Data curation, Writing – review & editing. Sandra Mechó: Methodology, Investigation, Data curation, Formal analysis, Writing – review & editing. Xavier Padulles: Investigation, Writing – review & editing. Josep Maria Padulles: Investigation, Writing – review & editing. Joan Aureli Cadefau: Conceptualization, Methodology, Investigation, Writing – original draft, Writing – review & editing, Project administration.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.