Eccentric Exercise (EE) can induce muscle damage, but ischemic preconditioning (IPC) emerge as a valuable approach to hasten the recovery process. Monitoring serum markers of muscle damage and assessing cellular health are essential to evaluate the effectiveness of this approach. Therefore, this study aimed to evaluate the behavior of cellular responses through bioelectrical impedance (BIA) vectors after IPC at different occlusion pressures and correlate them with indirect markers of eccentric muscle damage as creatine kinase (CK) and lactate dehydrogenase (LDH).
MethodsEighty men were divided into four groups: IPC with arterial occlusion pressure (AOP), IPC with 40 % above AOP, placebo, and control. All groups underwent an EE protocol and were evaluated for CK, LDH, and BIA. Repeated measures analysis of variance was used, and correlations were performed using Pearson or Spearman correlation depending on data normality. The significance level was set at p < 0.05.
ResultsThe placebo and control groups showed an increase in CK and LDH levels, along with a decrease in cellular integrity. The IPC-AOP group exhibited an increase in CK and LDH levels, but without negative impact on resistance, integrity, and cellular health. The IPC-40 % group showed an increase in CK, decrease in cellular health, elevation of LDH, and higher tissue electrical resistance.
ConclusionThe interventions were not effective in maintaining cellular health in the context of eccentric muscle damage.
The practice of eccentric exercise (EE) is prevalent in rehabilitation and fitness.1 Beyond its benefits,2 this modality, when performed unusually, induces muscular damage.1 Morphologically, damage can lead to sarcomere degeneration and Z-line fragmentation. Consequently, metabolically depleted fibers may exhibit decreased resistance in cell membranes,3 allowing for the understanding of intracellular extravasation.4,5
Instruments like creatine kinase (CK) and lactate dehydrogenase (LDH) concentrations are the most suitable methods to identify this type of damage. Elevated serum levels confirm the scenario of damage after exercise.6-9 Considering membranal damage, bioelectrical impedance (BIA)10–13 serves as a tool to evaluate cellular health and integrity. BIA facilitates the determination of resistance (R), reactance (Xc), and phase angle (phA) values.11
Physiologically, Xc values correspond to cellular membrane integrity, while R values reflect tissue hydration levels.11 On the other hand, phA is derived through the integration of these variables, functioning as an indicator of cellular membrane functionality.14 Lower phA values indicate diminished cellular integrity or cell death, associated with muscular injuries.15 Conversely, higher values may indicate a larger number of intact cellular membranes and an optimal state of health.15
Do elevated levels of CK and LDH signify compromised cellular health and integrity during and post EE? Are there viable alternatives to effectively mitigate such cellular damage? Strategies such as massage,16,17 cryotherapy,18 and compression garments19 are known for accelerating post-exercise recovery. Some pre-exercise strategies, including ischemic preconditioning (IPC),20 have been employed to minimize damage.
IPC is a technique involving periodic blood flow occlusion, inducing cycles of ischemia-reperfusion.21 This approach can be implemented with varied occlusion pressures to accelerate the recovery process post-exercise-induced muscle damage. The underlying mechanism involves enhancing blood flow by activating ATP-sensitive potassium channels, increasing adenosine levels, and/or attenuating the inflammatory response.22,23
Alterations arising from ischemia-reperfusion periods are linked to diverse cellular modifications within the musculoskeletal system, resembling those observed in eccentric muscle damage.20 Given the comparable pathogenesis between IPC and eccentric muscle damage, pre-exercise application of IPC can mitigate the extent of muscle damage and subsequent pre-inflammatory responses. Consequently, IPC has the potential to directly stimulate the organism's physiological defense systems and enhance tissue health and integrity.20,24
The present study aimed to evaluate cellular responses (R, Xc, and phA) following IPC at different occlusion pressures and correlate them with indirect markers of eccentric muscle damage (CK and LDH). The hypothesis posits that IPC will confer a protective effect against eccentric muscle damage. Additionally, these protective responses are expected to manifest prominently when examining indirect markers of cellular damage and phA, particularly in instances of higher occlusion pressure, given the resultant augmented blood flow and elevated post-ischemia adenosine levels.22,23
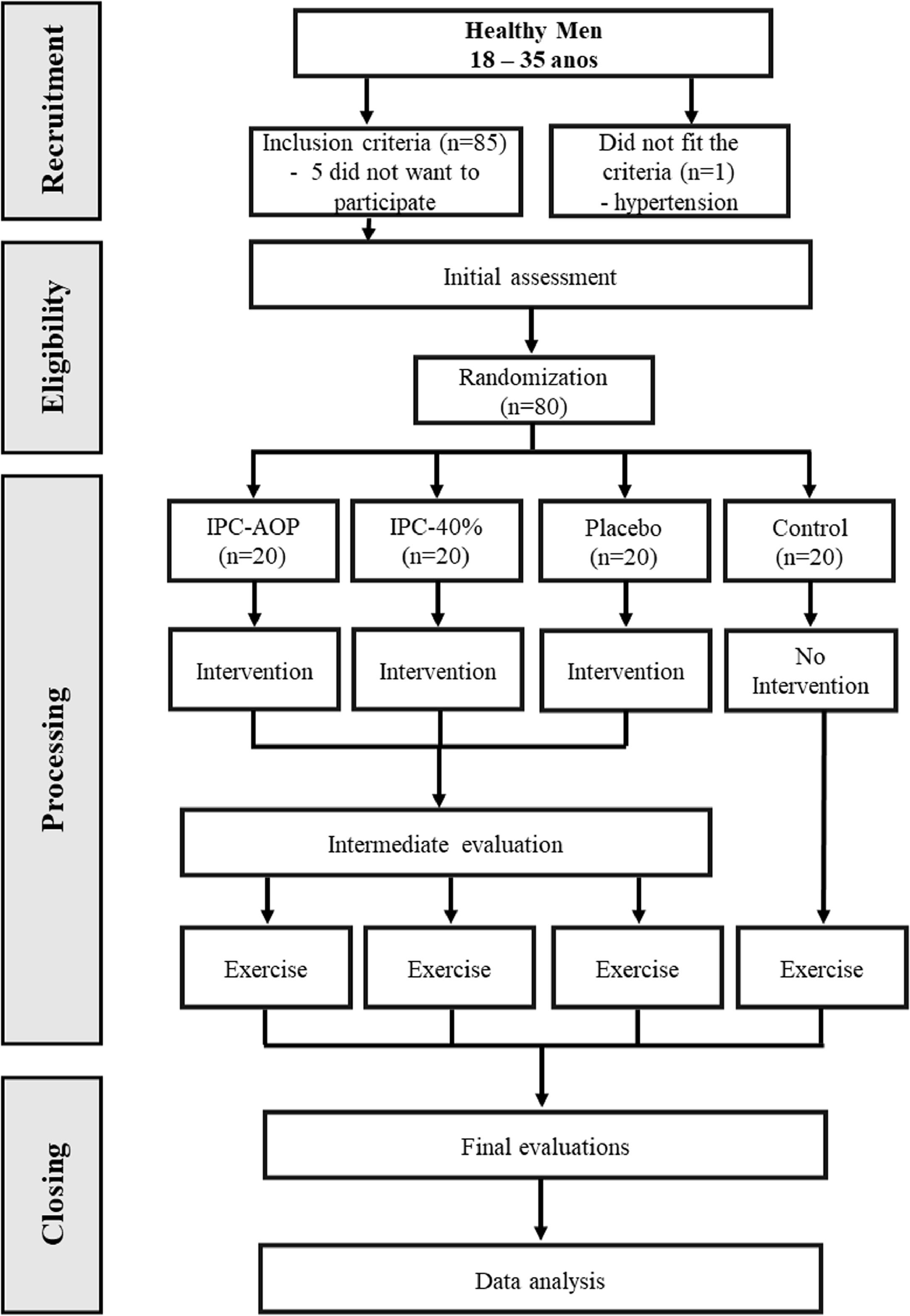
MethodsParticipants and ethics approvalHealthy male individuals, aged between 18 and 35 years, were recruited through a combination of laboratory database selection and social media advertising. The sample size determination was based on a prior study conducted by Bailey et al.,25 where a standard deviation of 200 μL in CK concentration was considered. A two-tailed hypothesis test with a test power of 80 % and a significance level of 5 % was employed, leading to a final sample size of 20 participants per group, resulting in a total of 80 participants.
Individuals presenting any of the following characteristics were excluded from participation: (1) presence of any health condition contraindicating or impeding EE; (2) diagnosed diabetes and arterial hypertension; (3) inflammatory rheumatological, psychiatric, cardiovascular, and/or respiratory diseases; (4) alcoholism, drug use, and/or smoking; (5) history of knee surgery (e.g., meniscal repair and ligament reconstruction) or recent musculoskeletal lower limb injury that could affect performance during tests or interventions (e.g., muscle injury, tendinopathy, patellofemoral pain in the lower limbs, and/or back pain in the past six months); (6) engagement in any type of training program during the study period; (7) participation in a lower limb strength training program in the three months prior to study enrollment; (8) use of ergogenic supplements to enhance physical performance and/or muscle mass and/or vasoactive medications; (9) presence of one or more predisposing risk factors for thromboembolism.26
Participants who expressed a voluntary intention to withdraw from the study, experienced a musculoskeletal injury episode during exercise that prevented further participation, did not adequately understand the execution of EE, or used any form of pain relief therapy during the study were excluded. However, there were no exclusions in the study.
The included participants were adequately informed about the procedures and objectives of this study, and upon their agreement, they signed an informed consent form, becoming actively involved in the study. The conduct of the study was approved by the local ethics committee (CAAE: 30765020.3.0000.5402). The study was prospectively registered (ClinicalTrials.gov; NCT04420819).
Study designThis is a four-arm, randomized, placebo-controlled, and single-blind study conducted at the Center for Studies and Rehabilitation in Physiotherapy (CEAFIR) of FCT/UNESP. The study design was based on the protocol by Pizzo Junior et al.,27 where each participant attended the clinic for five consecutive days.
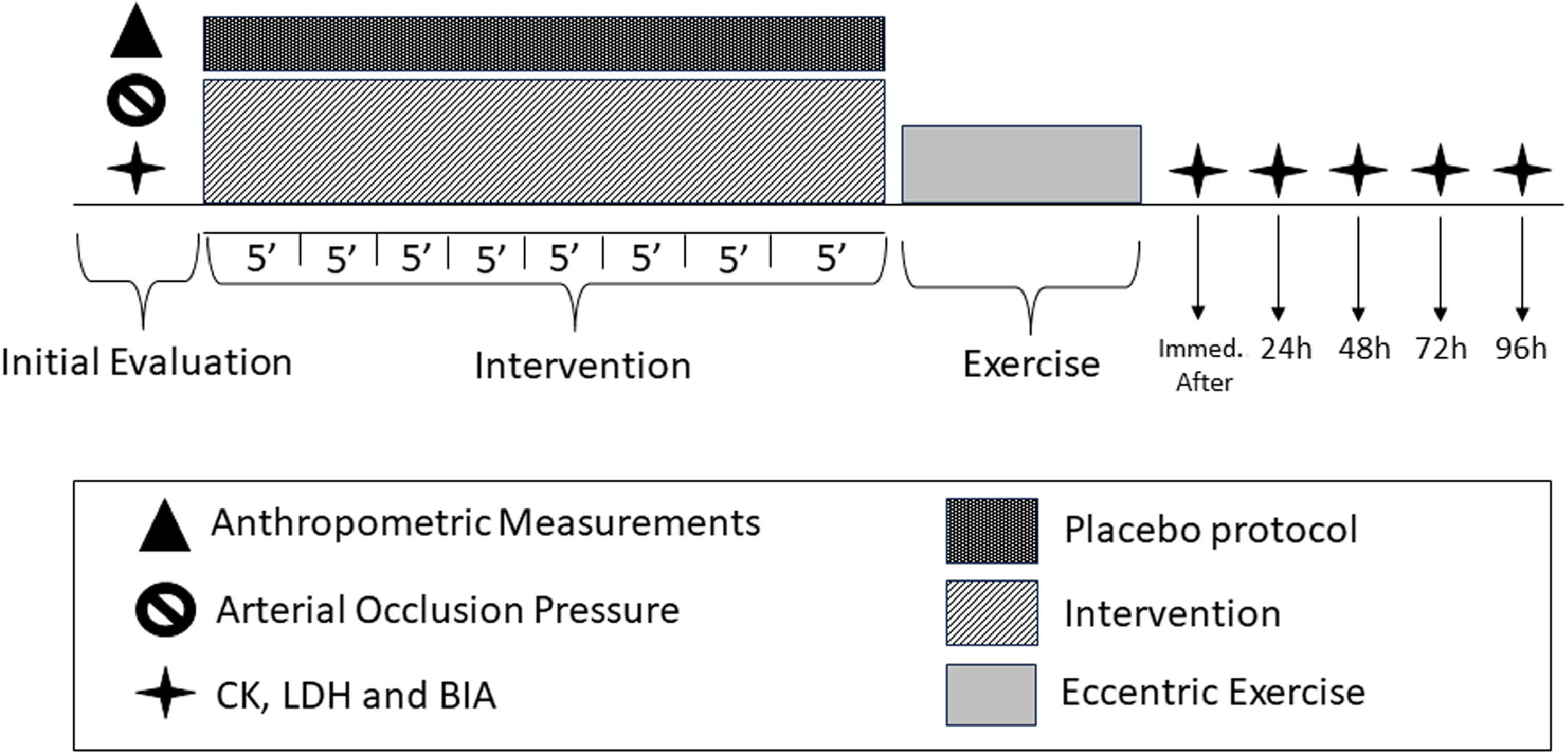
Initially, the participants underwent an evaluation of anthropometric measurements utilizing a scale (Tanita BC 554, Iron Man/Inner, Arlington Heights Illinois, USA) and a stadiometer (Sany - American Medical do Brasil, São Paulo, Brazil). Subsequently, the body mass index (BMI) was calculated. Afterward, the participants assumed a supine position and rested for 10 min, during which the assessment of total arterial occlusion pressure (AOP) took place. Subsequently, baseline evaluations of CK, LDH, and BIA were conducted.
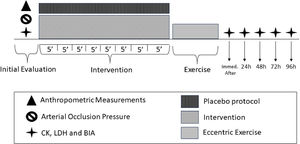
Subsequently, the participants underwent a previously randomized protocol of IPC or placebo lasting for 40 min. Following this intervention, the EE was initiated. Immediately after the completion of the EE, all outcome measures were collected once again. Subsequent visits were scheduled at 24, 48, 72, and 96 h after the EE, during which the same aforementioned outcome measures were collected, while maintaining the same order of assessment execution. The study design is illustrated in the Fig. 1.
Randomization and blindingThe participants were randomly allocated to i) control, remained at rest during the intervention period; ii) placebo, used 10 mmHg; iii) IPC using total occlusion pressure (PCI-AOP); and iv) IPC with 40 % more than the total occlusion pressure (IPC-40 %). Randomization was performed using a randomly generated numerical sequence on the website www.randomization.com. Participants allocation was concealed in sequentially numbered, opaque, sealed envelopes prepared before the study by a researcher not involved in the study. The evaluators who performed the outcome measurements were unaware of the allocation, and the statistician was also blinded. The therapist and participants were not blinded to the randomization due to the nature of the study.
ProceduresDetermination of AOPAOP was determined using a vascular Doppler (DF-7000V; Medpej, Ribeirão Preto, São Paulo, Brazil). All participants were instructed to avoid strenuous exercise and alcohol intake in the 48 h prior to the AOP assessment. Participants were placed in a supine position for 10 min in a climate-controlled room. Immediately after, the Doppler transducer was positioned on the posterior tibial artery (average distance between the medial malleolus of the tibia and the Achilles tendon), while the cuff (customized cuff, Velcro; 12.5 cm wide and 84 cm long, with an inflatable chamber 7 cm wide and 52 cm long, Cardiomed, Curitiba, Paraná, Brazil) was positioned around the subinguinal region on the medial portion of the thigh, covering the femoral artery.26 The cuff was manually inflated based on a previous protocol,28 and AOP was defined as the minimum pressure required to abolish the arterial pulse.
IPC and placebo protocolThe IPC protocol was applied to the inguinal region of the dominant limb with participants relaxed and comfortably positioned in a supine position. The same cuff used to determine AOP was used, and the protocol consisted of four cycles of ischemia (individually determined AOP) of five minutes, followed immediately by four cycles of five minutes of vascular reperfusion (0 mmHg), totaling 40 min.
For the implementation of the IPC protocol, one of the study groups used 40 % more than AOP, as according to the study by Lopes et al.,29 the pressure at which blood flow ceases to pass to the tibial artery ranges from 140 to 160 mmHg, and most studies in which IPC is performed use a pressure between 200 and 220 mmHg, thus the values are correspondent.29 The other study group used the exact AOP, as this value results in the absence of blood flow.
For the placebo group, a similar protocol was applied, however, four cycles of five minutes of placebo occlusion (10 mmHg) were alternated with four cycles of five minutes of reperfusion (0 mmHg).30,31 It is worth noting that in all three study groups, participants were informed in advance that the applied occlusion pressure would be sufficient to improve performance and prevent muscle damage.
Eccentric exercise protocolThe EE was performed on an isokinetic dynamometer (Biodex System 4 Pro, New York, USA) for the knee extensor muscles of the dominant limb. The protocol was based on the study by Machado et al.32 Initially, five submaximal knee extension contractions were performed to familiarize the participant with a speed of 60°/s (1.04 rad/s) and a range of motion of 60°. After five minutes, the muscle damage protocol was initiated, consisting of 5 sets of 15 maximum eccentric knee extension contractions, with 30 s of rest between sets, totaling 75 repetitions. The speed and range of motion were similar to the familiarization phase, and verbal encouragement was provided throughout the protocol.
Evaluated outcomesPhase angle and BIA vectorsAll participants were evaluated in a supine position on an electrically isolated stretcher, with legs abducted at a 45° angle. BIA assessment was performed using tetrapolar electrodes.33,34 For localized evaluation of the quadriceps femoris, the electrodes were positioned on the dominant hand and five centimeters from the anterosuperior iliac spine and above the superior pole of the patella. In global evaluation, the electrodes were positioned on the dominant hand (between the styloid processes of the radius and ulna and at the base of the phalanx of the 3rd metacarpal) and on the dominant foot (between the lateral and medial malleoli and at the base of the phalanx of the 3rd metatarsal).
Measurements of R and Xc were used to determine phA using the following equation: phA = arctangent (Xc/R) x (180°/π). The Bioscan program BL-960141 (Biologica, Barcelona, Spain)33,34 was used for the analyses. BIA measurements were collected at the following time points: baseline assessment, immediately after the completion of the EE, 24 h, 48 h, 72 h, and 96 h after the intervention.
CK concentrationThe CK concentration was obtained using 32 μL of capillary blood collected from the fingertip pulp. The blood sample was drawn into a heparinized capillary tube and then pipetted onto a CK test strip for analysis on the Reflotron Plus System (Roche Diagnostics, Mannheim, Germany) using the reflection photometry method at 37 °C (test temperature).35 CK measurements were collected at the following time points: baseline assessment, immediately after the completion of the EE, 24 h, 48 h, 72 h, and 96 h after the intervention.
LDH concentrationTo assess LDH concentration, 25 μL of blood was collected from the earlobe using a capillary tube. Heparinized capillaries and polyethylene Eppendorf tubes (1.5 mL) containing 50 μL of sodium fluoride (NaF - 1 %) were used. The analyses were performed using a lactometer (YSI, Yellow Springs - 2900),36 with values expressed in mmol/L. It is worth noting that LDH was collected at the following time points: pre-PCI protocol, pre-EE, immediately after the completion of EE at 1st, 3rd, 5th, 7th, 9th, 11th, 13th, and 15th minutes, 24 h, 48 h, 72 h, and 96 h after the intervention.
Statistical analysisData normality was assessed using the Kolmogorov-Smirnov test. Sample characteristics variables were presented as mean, standard deviation, median, and interquartile range. One-way ANOVA with Tukey's post hoc test or Kruskal-Wallis with Dunn's post hoc test, depending on data normality, were used to compare sample characteristics.
Correlations between outcomes were assessed using Pearson's or Spearman's correlation test, depending on data normality, to analyze possible associations between changes in phA, R, and Xc with changes in metabolic variables (CK and LDH).
Point-biserial correlation (expressed by r values) and its respective significance level indicated the relationship between metabolic responses and BIA, with the classification suggested by Portney and Watkins37: 0.00 to 0.19 for very weak correlation, 0.20 to 0.39 for weak correlation, 0.40 to 0.69 for moderate correlation, 0.70 to 0.89 for strong correlation, and 0.90 to 1.00 for very strong correlation.37 Statistical significance was set at values below 5 %. The statistical software SPSS (version 25) (SPSS Inc., Chicago, IL, USA) was used for the analyses.
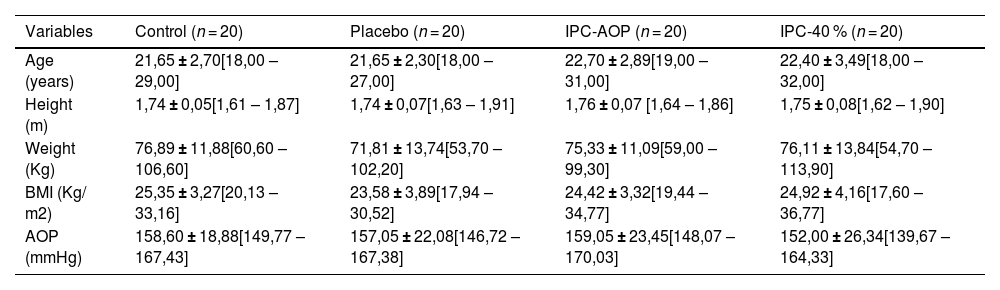
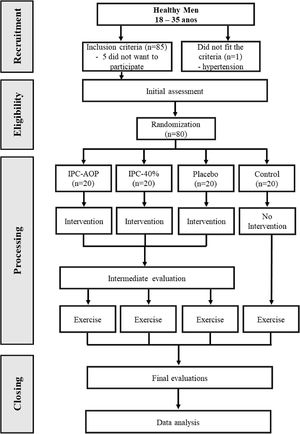
ResultsParticipants were recruited between June 2021 and March 2022. Participants characteristics are presented in Table 1. Demographic variables were similar at the beginning of the study. Eighty-six participants were assessed for eligibility, and 80 were included, with no exclusions during the study. The detailed flowchart is shown in Fig. 2.
Characteristics of participants of the study sample.
Values presented as mean ± SD [Minimum and maximum value].
Legenda: SD: standard deviation; AOP: Arterial occlusion Pressure; IPC-40 %: group with 40 % more than the total AOP; Control: which remained at rest during the intervention period; Placebo: which used 10 mmHg; IPC-AOP: Group using total occlusion pressure; BMI: body mass index; m: meters; Kg: kilograms; Kg.m2: kilogram per square meter.
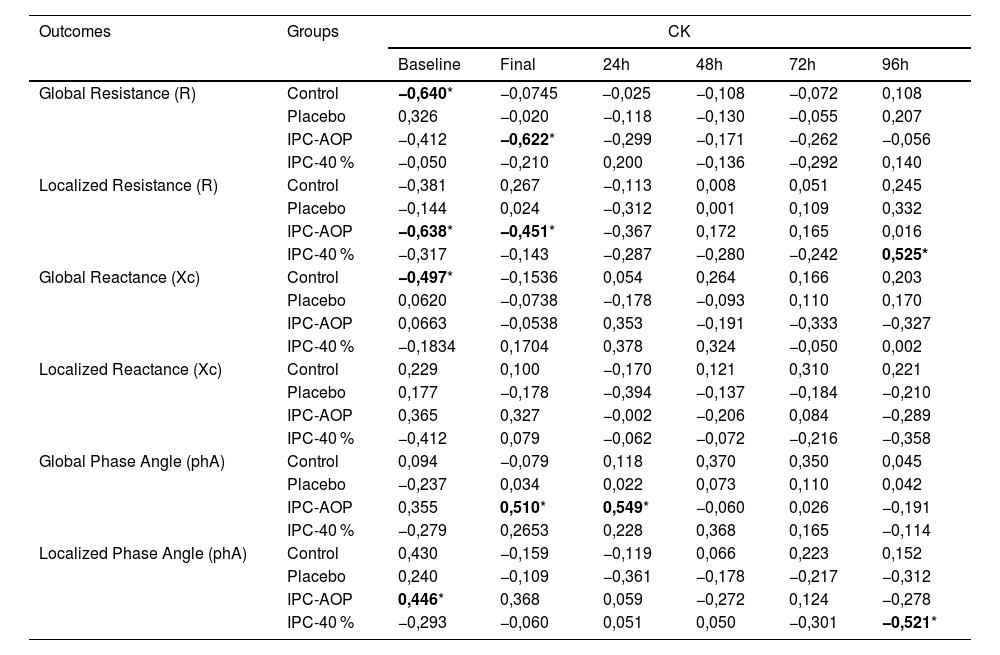
The comparative results among the studied groups of BIA, CK, and LDH are in the Appendices. Table 2 represents the correlation of phA and its vectors of global and localized bioimpedance with CK. For the IPC-AOP group, a significant moderate correlation was observed at baseline between CK and localized R (r=−0.638) and localized phA (r = 0.446), respectively negative and positive. Furthermore, there was also a moderate negative correlation at the final moment of CK with global R (r=−0.622) and localized R (r=−0.451), as well as a moderate positive correlation with global phA (r = 0.510). Finally, there was a moderate positive correlation at 24 h after the intervention between CK and global phA (r = 0.549). For the IPC-40 % group, a moderate correlation was observed at 96 h between CK and localized R (r = 0.525) and localized phA (r=−0.521). For the control group, there was a significant correlation between CK and global R (r=−0.640) and Xc (r=−0.497), both moderate and negative. No correlation was observed for the placebo group.
Correlation of cellular outcomes with CK.
| Outcomes | Groups | CK | ||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Final | 24h | 48h | 72h | 96h | |||
| Global Resistance (R) | Control | −0,640* | −0,0745 | −0,025 | −0,108 | −0,072 | 0,108 | |
| Placebo | 0,326 | −0,020 | −0,118 | −0,130 | −0,055 | 0,207 | ||
| IPC-AOP | −0,412 | −0,622* | −0,299 | −0,171 | −0,262 | −0,056 | ||
| IPC-40 % | −0,050 | −0,210 | 0,200 | −0,136 | −0,292 | 0,140 | ||
| Localized Resistance (R) | Control | −0,381 | 0,267 | −0,113 | 0,008 | 0,051 | 0,245 | |
| Placebo | −0,144 | 0,024 | −0,312 | 0,001 | 0,109 | 0,332 | ||
| IPC-AOP | −0,638* | −0,451* | −0,367 | 0,172 | 0,165 | 0,016 | ||
| IPC-40 % | −0,317 | −0,143 | −0,287 | −0,280 | −0,242 | 0,525* | ||
| Global Reactance (Xc) | Control | −0,497* | −0,1536 | 0,054 | 0,264 | 0,166 | 0,203 | |
| Placebo | 0,0620 | −0,0738 | −0,178 | −0,093 | 0,110 | 0,170 | ||
| IPC-AOP | 0,0663 | −0,0538 | 0,353 | −0,191 | −0,333 | −0,327 | ||
| IPC-40 % | −0,1834 | 0,1704 | 0,378 | 0,324 | −0,050 | 0,002 | ||
| Localized Reactance (Xc) | Control | 0,229 | 0,100 | −0,170 | 0,121 | 0,310 | 0,221 | |
| Placebo | 0,177 | −0,178 | −0,394 | −0,137 | −0,184 | −0,210 | ||
| IPC-AOP | 0,365 | 0,327 | −0,002 | −0,206 | 0,084 | −0,289 | ||
| IPC-40 % | −0,412 | 0,079 | −0,062 | −0,072 | −0,216 | −0,358 | ||
| Global Phase Angle (phA) | Control | 0,094 | −0,079 | 0,118 | 0,370 | 0,350 | 0,045 | |
| Placebo | −0,237 | 0,034 | 0,022 | 0,073 | 0,110 | 0,042 | ||
| IPC-AOP | 0,355 | 0,510* | 0,549* | −0,060 | 0,026 | −0,191 | ||
| IPC-40 % | −0,279 | 0,2653 | 0,228 | 0,368 | 0,165 | −0,114 | ||
| Localized Phase Angle (phA) | Control | 0,430 | −0,159 | −0,119 | 0,066 | 0,223 | 0,152 | |
| Placebo | 0,240 | −0,109 | −0,361 | −0,178 | −0,217 | −0,312 | ||
| IPC-AOP | 0,446* | 0,368 | 0,059 | −0,272 | 0,124 | −0,278 | ||
| IPC-40 % | −0,293 | −0,060 | 0,051 | 0,050 | −0,301 | −0,521* | ||
Legenda: IPC-40 %: group with 40 % more than the total AOP; Control: which remained at rest during the intervention period; Placebo: which used 10 mmHg; IPC-AOP: Group using total occlusion pressure; CK: creatine kinase.
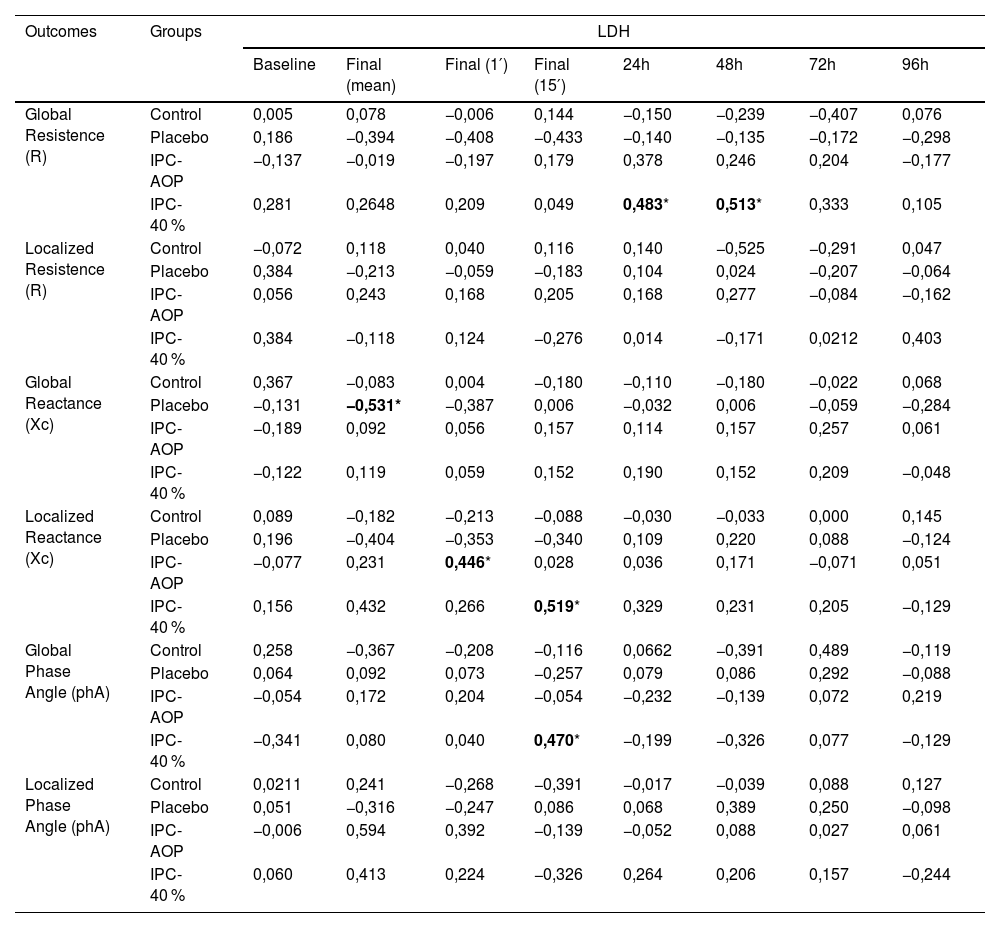
Table 3 represents the correlation of phA and its vectors of global and localized bioimpedance with LDH. For the IPC-40 % group, a significant correlation was observed at 24 and 48 h after the intervention between LDH and global R (r = 0.483 and r = 0.513, respectively), as well as at the final moment of 15 min of LDH with localized Xc (r = 0.519) and global phA (r = 0.470), all being positive and moderate. For the IPC-AOP group, a moderate positive correlation was observed in the 1st minute immediately after the exercise between LDH and localized Xc (r = 0.446). For the placebo group, there was a moderate negative correlation for the average of the LDH collection moments immediately after the exercise with global Xc (r=−0.531). All other correlations were not significant.
Correlation of cellular outcomes with LDH.
| Outcomes | Groups | LDH | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Final (mean) | Final (1′) | Final (15′) | 24h | 48h | 72h | 96h | ||||
| Global Resistence (R) | Control | 0,005 | 0,078 | −0,006 | 0,144 | −0,150 | −0,239 | −0,407 | 0,076 | ||
| Placebo | 0,186 | −0,394 | −0,408 | −0,433 | −0,140 | −0,135 | −0,172 | −0,298 | |||
| IPC-AOP | −0,137 | −0,019 | −0,197 | 0,179 | 0,378 | 0,246 | 0,204 | −0,177 | |||
| IPC-40 % | 0,281 | 0,2648 | 0,209 | 0,049 | 0,483* | 0,513* | 0,333 | 0,105 | |||
| Localized Resistence (R) | Control | −0,072 | 0,118 | 0,040 | 0,116 | 0,140 | −0,525 | −0,291 | 0,047 | ||
| Placebo | 0,384 | −0,213 | −0,059 | −0,183 | 0,104 | 0,024 | −0,207 | −0,064 | |||
| IPC-AOP | 0,056 | 0,243 | 0,168 | 0,205 | 0,168 | 0,277 | −0,084 | −0,162 | |||
| IPC-40 % | 0,384 | −0,118 | 0,124 | −0,276 | 0,014 | −0,171 | 0,0212 | 0,403 | |||
| Global Reactance (Xc) | Control | 0,367 | −0,083 | 0,004 | −0,180 | −0,110 | −0,180 | −0,022 | 0,068 | ||
| Placebo | −0,131 | −0,531* | −0,387 | 0,006 | −0,032 | 0,006 | −0,059 | −0,284 | |||
| IPC-AOP | −0,189 | 0,092 | 0,056 | 0,157 | 0,114 | 0,157 | 0,257 | 0,061 | |||
| IPC-40 % | −0,122 | 0,119 | 0,059 | 0,152 | 0,190 | 0,152 | 0,209 | −0,048 | |||
| Localized Reactance (Xc) | Control | 0,089 | −0,182 | −0,213 | −0,088 | −0,030 | −0,033 | 0,000 | 0,145 | ||
| Placebo | 0,196 | −0,404 | −0,353 | −0,340 | 0,109 | 0,220 | 0,088 | −0,124 | |||
| IPC-AOP | −0,077 | 0,231 | 0,446* | 0,028 | 0,036 | 0,171 | −0,071 | 0,051 | |||
| IPC-40 % | 0,156 | 0,432 | 0,266 | 0,519* | 0,329 | 0,231 | 0,205 | −0,129 | |||
| Global Phase Angle (phA) | Control | 0,258 | −0,367 | −0,208 | −0,116 | 0,0662 | −0,391 | 0,489 | −0,119 | ||
| Placebo | 0,064 | 0,092 | 0,073 | −0,257 | 0,079 | 0,086 | 0,292 | −0,088 | |||
| IPC-AOP | −0,054 | 0,172 | 0,204 | −0,054 | −0,232 | −0,139 | 0,072 | 0,219 | |||
| IPC-40 % | −0,341 | 0,080 | 0,040 | 0,470* | −0,199 | −0,326 | 0,077 | −0,129 | |||
| Localized Phase Angle (phA) | Control | 0,0211 | 0,241 | −0,268 | −0,391 | −0,017 | −0,039 | 0,088 | 0,127 | ||
| Placebo | 0,051 | −0,316 | −0,247 | 0,086 | 0,068 | 0,389 | 0,250 | −0,098 | |||
| IPC-AOP | −0,006 | 0,594 | 0,392 | −0,139 | −0,052 | 0,088 | 0,027 | 0,061 | |||
| IPC-40 % | 0,060 | 0,413 | 0,224 | −0,326 | 0,264 | 0,206 | 0,157 | −0,244 | |||
Legenda: IPC-40 %: group with 40 % more than the total AOP; Control: which remained at rest during the intervention period; Placebo: which used 10 mmHg; IPC-AOP: Group using total occlusion pressure; LDH: lactate dehydrogenase; Mean: The sum of the eight time points of lactate divided by eight; 1′: values of the first minute of lactate; 15′ values of 15 min of lactate.
The objective of the present study was to evaluate cellular responses (R, Xc, and phA) after IPC at different occlusion pressures, correlating them with indirect markers of eccentric muscle damage (CK and LDH). Initial hypotheses suggested IPC would provide a protective effect against eccentric muscle damage, especially at higher occlusion pressures, with increased blood flow and post-ischemic adenosine.22,23
However, the results obtained in the present study did not fully confirm the hypotheses. The placebo and control groups showed an increase in CK and LDH associated with a reduction in cellular integrity, demonstrating that EE induced metabolic changes altering the vector of BIA. On the other hand, the IPC-AOP group showed an increase in CK and LDH levels; however, these did not negatively affect resistance, cellular integrity, and health, indicating that EE did not exert its damaging role on the cellular parameters. The physiological effects justifying this increase in CK and LDH levels are not fully understood. However, the IPC-40 % group exhibited an increase in CK levels accompanied by a decrease in cellular health and lower conductive capacity, as well as an increase in LDH associated with an increase in tissue electrical resistance, representing a worsening of responses.
The utilization of CK and LDH as physiological markers in recreational and sports settings has been extensively documented in existing literature.38 This leads to inquiries regarding the validation of phA and BIA vectors as indicators of muscle damage. In a comprehensive review conducted by Castizo-Olier et al.,39 various sources pertaining to the application of BIA in sports and exercise were systematically examined to assess its effectiveness in these contexts. Notably, within the domain of injury identification and monitoring, the review identified two studies 40,41 that observed a reduction in R and Xc values within injured muscles, attributable to factors such as edema and muscle structure rupture respectively. Consequently, the authors concluded that localized vector analysis derived from BIA presents itself as a viable alternative approach that can aid in evaluating soft tissue injury.39
To some extent, the vectorial results obtained from the mentioned studies appear to support the observed correlations, particularly within the placebo and control groups, where tissue damage is indeed present without any intervention. However, when analyzing the outcomes of the IPC-AOP and IPC-40 % groups, contrasting results emerge. The findings from the IPC-AOP group indicate that the application of this technique did not sustain the detrimental effects of EE on cellular health, as evidenced by the absence of negative repercussions on resistance, integrity, and cellular health. Conversely, the IPC-40 % group exhibited an elevation in the serum concentration of metabolic markers and a deterioration in cellular responses. Nevertheless, it is not plausible to disregard the potential interaction of these responses with IPC.
Initially, the dose-response relationship resulting from the application of various occlusion pressures can be considered a crucial factor in comprehending the findings of the current study. One factor substantiating this relationship is the likely presence of a non-linear dynamic behavior in the CK and LDH variables. As elucidated by De Garcia,42 total damage and cellular responses to stress undergo temporal changes contingent upon the magnitude of the injury. Consequently, the non-linear orders are defined by systems that are susceptible and prone to perturbation factors,43 including time and magnitude.
Hence, when providing a comprehensive discussion of the findings, the distinct correlations observed within each group can be rationalized. It is recognized that varying the occlusion pressure can induce alterations in the metabolic and cellular variables, underscoring the dose-response relationship.
Results like these demonstrate the complexity involved in analyzing variables that indicate cellular damage. It is known that various systems are disrupted during EE, and manifestations of cellular damage can occur through multifactorial mechanisms. Therefore, it is not disregarded that future research should continue to investigate physiological responses following IPC, using different application protocols and analyzing them through novel tools. This would enable the exploration of relevant outcomes for understanding the technique, introducing new elements to the pre-exercise recovery literature and potentially enabling its clinical application to expedite the recovery process.
The present study is limited by the fact that the methodological protocols were conducted solely on the dominant limb, thus hindering intra-individual comparisons. Consequently, data for comparing a homeostatic condition with the effects of muscle stress during subsequent assessment moments following the intervention could not be obtained.
The most relevant findings in the present study were the correlations observed in the IPC-40 % group. Although the IPC-AOP group showed favorable results in terms of cellular integrity, the values of metabolic variables were contradictory, highlighting the need for studies that can address the non-linear nature of these variables. Therefore, even though the IPC-40 % group exhibited a non-protective outcome in terms of CK, LDH, and BIA results, their findings can be interpreted with more certainty. The application of IPC did not exacerbate detrimental effects on cellular responses, but it also did not demonstrate a protective effect on post-EE responses. Consequently, when compared to the results of the control and placebo groups, these data indicate that the clinical application of this intervention does not appear to be an effective alternative for cellular health in the context of eccentric muscle damage.
ConclusionIt can be concluded that moderate correlations were found between cellular responses (R, Xc, and phA) and indirect markers of eccentric muscle damage (CK and LDH) following IPC at different occlusion pressures. The placebo, control, and IPC-40 % groups demonstrated an increase in markers of muscle damage associated with a worsening of cellular responses. Despite the IPC-POT group showing results closer to our hypotheses, the application of these interventions does not appear to be an alternative for maintaining cellular health in the context of eccentric muscle damage.
This study was carried out with the support of the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) – number 2021/11893-2.