To review the current scientific evidence for the clinical use of percutaneous needle electrolysis (PNE) in musculoskeletal conditions.
MethodsA systematic electronic search was performed in biomedical databases. Only clinical studies on human subjects using PNE on musculoskeletal pathologies were included. Methodological quality and risk of bias were assessed using the methodological index for non-randomized studies (MINORS). Treatment protocols were described, and primary outcomes (pain, injury-related function, and tissue structure) were compared against other treatment modalities or control groups in short (<1 month), mid (1-3 months) and long term (>3 months).
ResultsTwenty-one studies met eligibility criteria (14 comparative studies and 7 case series). Sixty-two percent were at moderate to high risk of bias. PNE was applied in a wide range of injury types (mostly tendon-related), and application protocols were heterogeneous in terms of dosage (intensity: 0.35-6mA; time: 9-90sec), frequency (from twice a week to once every 2 weeks) and treatment duration (1-10 weeks). PNE showed moderate effects on pain at short and mid-term compared to active exercise interventions alone and sham needling. There is limited evidence that PNE improves injury-related function compared to other treatment modalities and no evidence of tissue structure improvement after PNE application.
ConclusionThere is paucity of high-quality clinical studies about PNE in musculoskeletal conditions and lack of consensus about treatment indications and application protocols. Although a moderate effect on pain at short and mid-term has been documented, further research is needed.
Ultrasound-guided percutaneous needle electrolysis (PNE) is an invasive technique used for the treatment of musculoskeletal injuries that has gained popularity among physiotherapists over the last few years. The technique consists of the application of a high-intensity galvanic continuous electrical current to the injured area by means of an ultrasound-guided acupuncture needle. This induces a local inflammatory response through the cathodic flow which facilitates the phagocytosis of the damaged cells and the regeneration of damaged tissue.1
The application of galvanic current to tendons was already documented by Owoeye in 19872 but it was Sánchez-Ibáñez who popularized the technique with its use in chronic patellar tendinopathy during the first decade of 2000.1 Although the literature about PNE is scarce, several studies have tried to establish its mechanism at the molecular level in tendons using animal models. Abat et al (2014)3 investigated the mechanisms of PNE in collagenase-induced tendinopathy in rats, showing increased levels of proteins inducing cell apoptosis (Cytochrome C and Smac/Diablo), anti-inflammatory mediators (peroxisome gamma proliferator activator [PPAR-gamma]) and angiogenic responses (vascular endothelial growth factors VEGF and VEGF-R1). Sanchez-Sanchez et al. (2020)4 found an increased expression of some genes associated with collagen regeneration and remodeling of extracellular matrix in collagenase-induced achilles tendinopathy when compared to sham needling in an experimental animal model. More recently, Penin-Franch et al. (2022)5 described that the galvanic current may induce an inflammatory response that promotes a collagen-mediated regeneration of the tendon in mice. However, there are conflicting opinions regarding studies on tendinopathy in animal models6 as they may not reflect the real pathophysiology of the injured human tendon.
The potential mechanisms of PNE on muscle injuries were also investigated by Abat et al. in 20157 in a mouse model, showing that PNE may affect the inflammatory mediators and the neovascularization of the injured area, decreasing plasma levels of pro-inflammatory tumoral necrosis factor (TNF-alpha) and interleukin 1 (IL-1beta), and increasing anti-inflammatory mediators (PPAR-gamma) and the expression of proangiogenic factors VEGF and VEGF-R1.
Although initially indicated and used for chronic patellar tendinopathy8–10 the application of PNE evolved quickly and became popular among physiotherapists. Many studies emerged investigating its effects on pain, tissue repair and accelerated function recovery on a wide range of pathologies: epicondylalgia,11–13 carpal tunnel syndrome,14 proximal hamstring tendinopathy,15 hamstring and rectus femoris muscle injuries16,17 plantaris tendon disorder,18 achilles tendinopathy19,20 chronic soleus injuries,21 mammary fistula,22,23 groin pain,24,25 whiplash syndrome,26 temporomandibular myofascial pain,27,28 subacromial pain syndrome,29–31 or patellofemoral pain syndrome.32 However, many of those studies were case reports or case series studies without a control group, and there were only a few randomized control trials comparing PNE with other treatment modalities or with control groups. Other authors investigated the cost-effectiveness of PNE compared with conventional treatments33,34 as well as its safety,35 adverse effects,36 and potential effect on the autonomic response of the nervous system.37,38
Regarding the application protocols, there are also differences between authors: Sánchez-Ibáñez initially recommended intensities between 4 and 6 milliamperes in bouts of 3 to 5 seconds,39 but a number of studies13,29,30,40 applied lower intensities (0.35 milliamperes) and longer times (up to 1.2min) for musculoskeletal injuries. Specific indications about optimal dosages for different pathologies and/or injury stages (e.g., acute vs chronic) are still lacking, as well as studies comparing the effects of different dosages at the tissue level.
The aim of this study is to carry out a systematic review of the scientific literature about the use of PNE in musculoskeletal injuries, its indications, clinical effects, and existing protocols in order to identify possible gaps and homogenize its use in practice.
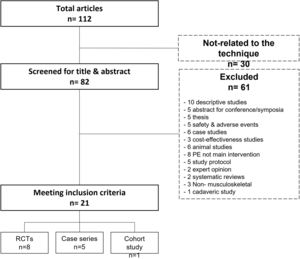
MethodsThe review was conducted in accordance with the international PRISMA guidelines.41 A literature search was carried out by two authors (DMS and FSM), who independently reviewed medical literature using biomedical databases (Medline, Sciencedirect, Dialnet, PEDro) and Google Scholar. The string of keywords used were: “percutaneous intratissue electrolysis”, “percutaneous needle electrolysis”, “ultrasound-guided electrolysis”, “ultrasound-guided galvanic electrolysis technique”, and “percutaneous electrochemical debridement”, fittingly connected by Boolean descriptors ("or" and "and"). Cross-references from included studies were also checked for relevance and included if meeting inclusion criteria. The search was conducted between 1st and 31st January 2022. No specific time or language filters were set. The PRISMA flow diagram of study search and selection is shown in Fig. 1.
Inclusion criteriaInclusion criteria according to the PICO acronym (Participants, Interventions, Comparisons, Outcomes) were P; clinical studies investigating the effects of PNE on human subjects. I; application of PNE on musculoskeletal pathologies. C; comparison with a control group, with other types of conservative treatement, or pre-post intervention within the same group. O; primary outcomes were pain, injury-related function and tissue structure. Other secondary outcomes also explored when available. Descriptive studies, case reports, experimental animal studies, conference abstracts and doctoral theses were excluded from the analysis, as well as those that applied the technique in laboratory settings, non-musculoskeletal pathologies or with other primary outcomes than those defined. For all articles meeting the inclusion criteria, relevant information was extracted and organized in a separate Excel Spreadsheet.
Data extraction synthesis and quality assessmentA descriptive analysis of the included studies was carried out (Table 3), including: reference, year, study design, injury type, study subjects’ characteristics, intervention, control group description, follow up details, outcome measures description and reported effects.
Follow-up periods were categorized as short-term (less than 1 month), mid-term (1–3 months), and long-term (>3 months) if the data were available.
The methodological index for non-randomized studies (MINORS criteria)42 was used to assess the methodological quality of the studies. A total score was provided based on a set of criteria to assess the methodological quality (8 items for non-comparative studies and 12 for comparative studies). Each item scored 0 if not reported; 1 when reported but inadequate; and 2 when reported and adequate. The maximum score was 16 for non-comparative studies and 24 for comparative studies.
For the risk of bias assessment, studies were considered at low risk of bias if achieving >75% in the MINORS score, at moderate risk if scoring 60 to 74%, and at high risk if <60%.43
Comparison with other treatment modalitiesFor comparative studies, PNE effects on the primary outcome measures (pain, injury-related function and tissue structure) was weighed against other treatment modalities or control groups when available. PNE was considered superior if it showed a significant positive effect compared to other treatment modalities/control groups, inconclusive if unclear positive effect (not in all reported measures or not clinically relevant), or not superior if no statistical differences were found.
ResultsStudy design and participantsA total of 112 articles were found at the initial search, 91 of which were excluded due to several reasons (Fig. 1). Twenty-one articles met all inclusion criteria and were finally selected for further analysis (14 comparative and 7 non-comparative studies).
The total sample included in the review was 954 subjects, of which 22.2% were athletes (n=212) and 78.8% non-athletes (n=742). A total of 629 subjects received PNE treatment (RCTs= 396; case series= 201; comparative= 32), while 377 were part of control groups (RCTs= 345; comparative= 32).
The most studied pathology was patellar tendinopathy (5 studies; 217 subjects included), followed by shoulder rotator cuff tendinopathy/subacromial pain syndrome (4 studies; 162 subjects), plantar fasciitis (3 studies; 233 subjects), groin pain (2 studies; 32 subjects), epicondylitis (2 studies; 68 subjects) and a variety of other pathologies with 1 study (whiplash syndrome, temporomandibular pain, acute rectus femoris muscle injury, achilles tendinopathy and chronic soleus injury).
Methodological quality and risk of biasStudy types, MINORS score, risk of bias and potential conflict of interest for all studies is described in Table 1. Eight studies are considered at low risk of bias, six at moderate risk, and seven at high risk. One study declared a potential conflict of interest.47 and eleven did not disclose any information about potential conflicts.
MINORS score, risk of bias and conflict of interest declaration for the studies included. MINORS maximal score= 24 for comparative studies and 16 for non-comparative studies.
| Author | Year | TYPE | MINORS Score | % | RISK OF BIAS | CONFLICT OF INTEREST |
|---|---|---|---|---|---|---|
| Valera44 | 2010 | Case series | 8/16 | 50% | HIGH | NONE |
| Abat45 | 2014 | Case series | 11/16 | 68% | MODERATE | N/A |
| Valera12 | 2014 | Case series | 10/16 | 63% | MODERATE | N/A |
| Abat10 | 2014 | Case series | 8/16 | 50% | HIGH | N/A |
| Arias Buria30 | 2015 | RCT | 21/24 | 88% | LOW | NONE |
| Moreno31 | 2016 | RCT | 17/24 | 71% | MODERATE | NONE |
| Abat46 | 2016 | RCT | 18/24 | 75% | MODERATE | N/A |
| Moreno24 | 2016 | Case series | 9/16 | 56% | HIGH | N/A |
| Garcia Naranjo26 | 2017 | RCT | 21/24 | 88% | LOW | N/A |
| Moreno25 | 2017 | RCT | 17/24 | 71% | MODERATE | N/A |
| De Miguel Valtierra29 | 2018 | RCT | 16/24 | 67% | MODERATE | NONE |
| Fernandez-Rodriguez47 | 2018 | RCT | 21/24 | 88% | LOW | YES |
| Iborra-Marcos48 | 2018 | Retrospective | 8/16 | 50% | HIGH | NONE |
| Lopez-Martos27 | 2018 | RCT | 21/24 | 88% | LOW | NONE |
| Al Boloushi49 | 2020 | RCT | 22/24 | 92% | LOW | N/A |
| Rodriguez-Huguet40 | 2020 | RCT | 22/24 | 92% | LOW | NONE |
| Rodriguez-Huguet13 | 2020 | RCT | 23/24 | 96% | LOW | NONE |
| Valera-Garrido17 | 2020 | Case series | 9/16 | 56% | HIGH | NONE |
| Calderon-Diez20 | 2020 | Case series | 8/16 | 50% | HIGH | N/A |
| De la Cruz21 | 2020 | RCT | 12/24 | 50% | HIGH | N/A |
| Lopez-Royo50 | 2021 | RCT | 24/24 | 100% | LOW | N/A |
Study protocols were very heterogenous. Only eight studies used PNE in isolation for the intervention group, and the vast majority combined PNE with different types of active exercise interventions (Table 3).
Seven studies compared PNE with other needle interventions like corticosteroid injections,48 placebo punctures47,27,50 or dry needling.27,50,40,13,49
Regarding the PNE application parameters, there were also differences in terms of intensity, time, frequency, and duration of the treatment (Table 2).
PNE application protocol. mA: milliamperes; sec: seconds; w: weeks; d: days.
| AUTHOR | YEAR | PARAMETERS Intensity Time | NEEDLE (Diameter x length) | FREQUENCY | Duration |
|---|---|---|---|---|---|
| Valera44 | 2010 | 4-6mAN/A | N/A | 1/week | Up to 6 weeks |
| Abat45 | 2014 | 3mAN/A | 0.30-0.32mm | 1/week | 1 to 10 weeks(Avg: 4.5w) |
| Valera12 | 2014 | 4-6mA3 sec, 3 times | 0.3 × 25mm | 1/week | 4 to 6 weeks |
| Abat10 | 2014 | 3mA3 times | 0.3mm | 1/bi-weekly | 1 to 10 weeks(Avg: 7.5w) |
| Arias Buria30 | 2015 | 0,35mA1.2 min | 0.3mm | 1/week | 4 weeks |
| Moreno31 | 2016 | 6mA4 sec, 3 times | N/A | 1/week | 3 weeks |
| Abat46 | 2016 | 2mA3 times | 0.25 × 25mm | 1/bi-weekly | Up to 2 months(Avg: 3.2 sessions) |
| Moreno24 | 2016 | 3mA4 sec, 3 times | 0.25 × 30mm | N/A | 2 to 6 sessions(Avg: 18d) |
| Garcia Naranjo26 | 2017 | 2-4mA3 sec, 3 times | 0.25 × 16mm | 1/week | 3 weeks |
| Moreno25 | 2017 | 3mA5 sec, 3 times | 0.33 × 50mm | 2/week | During Phase 1(Avg: 12d) |
| De Miguel Valtierra29 | 2018 | 0.35mA90 sec total | 0.3 × 25mm | 1/week | 5 weeks |
| Fernandez-Rodriguez47 | 2018 | 28 mC cathodalN/A | 0.35 × 40mm | 1/week | 5 weeks |
| Iborra-Marcos48 | 2018 | 3mA3 sec | G32 needle | 1/week | Up to 10 weeks(Avg: 5.2w) |
| Lopez-Martos27 | 2018 | 2mA3 sec, 3 times | 0.25 × 40mm | 1/week | 3 weeks |
| Al Boloushi49 | 2020 | 1.5mAN/A | 0.25-0.30 × 30-75mm | 1/week | 4 weeks |
| Rodriguez-Huguet40 | 2020 | 0,35mA1.2 min | N/A | 1/week | 4 weeks |
| Rodriguez-Huguet13 | 2020 | 0,35mA1.2 min | 0.3mm | 1/week | 4 weeks |
| Valera-Garrido17 | 2020 | 1.5-2mA3 sec, 5 times | 0.30 × 30mm | Once | Once at 48h |
| Calderon-Diez20 | 2020 | 3mA10sec | 0.3mm | 1/week | AVG: 5.9±4 sessions |
| De la Cruz21 | 2020 | 2.5mA3 sec, 3 times | 0.30 × 40mm | 1/week | 2 weeks |
| Lopez-Royo50 | 2021 | 3mA3 sec, 3 times | 0.25 × 25mm | 1/bi-weekly | 8 weeks |
A summary of all outcome measures, reported effects, and follow up periods is presented in Table 3. There was a high variability in the follow up periodization: 67% of the studies followed the subjects in the short term (<1 month), 76% in the mid-term (1-3 months) and 48% in the long term (>3 months); only 5 studies followed them in short, mid and long-term together.
Summary of findings for all studies included in the review. CG= Control group. IG= Intervention group.
| AUTHOR | YEAR | STUDY TYPE | INJURY TYPE (Target tissue) | STUDY SUBJECTS N (male/female) Type | INTERVENTION GROUP (IG) | CONTROL GROUP (CG) | FOLLOW-UP | OUTCOME MEASURES | REPORTED EFFECT |
|---|---|---|---|---|---|---|---|---|---|
| Valera44 | 2010 | CASE SERIES | Patellar tendinopathy(Proximal patellar tendon) | 32 (19/13)50% semi/ professional Athletes | IG: PNE + Eccentric exercises + stretchingn=32 | N/A | - Pre- Short-term: at discharge- Mid-term: n/a.- Long term: n/a | VISA-P | Improved at short-term. |
| TENDON STRUCTURE (US) | No significant changes. | ||||||||
| Abat45 | 2014 | CASE SERIES | Patellar tendinopathy (Proximal patellar tendon) | 33 (29/4)12% Prof. athletes67% semi-prof21% amateur | IG: PNE + Eccentric exercisesn=33 | N/A | - Pre- Short-term: n/a- Mid-term: 3 mo.- Long term: 2y | VISA P | Improved at 3mo. Maintained at 2y. |
| TEGNER SCORE | No significant changes. | ||||||||
| SATISFACTION SCALE | 79% excellent at 3mo.88% excellent at 2y. | ||||||||
| Valera12 | 2014 | CASE SERIES | Lateral epicondylitis (Common extensor tendon/extensor carpi radialis brevis) | 36 (19/17)Non-athletes(75% practiced upper body dominant sports) | IG: PNE + Ecentric exercises + Stretchingn=36 | N/A | - Pre- Short-term: at discharge (4w)- Mid-term: 6w- Long term: *26w, 52w (only patient perception of success) | DASH | Improved at short and mid-term (6w). |
| PAIN | Improved at short and mid-term (6w). | ||||||||
| PRESSURE PAIN | Improved at short-term/maintained at mid-term (6w). | ||||||||
| TENDON STRUCTURE (US) | ↓↓ vascularization mid-term (6w)↓ hypoechogeneity mid-term (6w)No change in thickness | ||||||||
| Perception of success | 83% successful at 6w.100% successful at 26 and 52w. | ||||||||
| Abat10 | 2014 | CASE SERIES | Patellar tendinopathy (Patellar tendon) | 40 (35/5)12.5% Prof. athletes67.5% semi-prof21% amateur | IG: PNE + Eccentric exercisesn=40 | N/A | - Pre- Short-term: n/a- Mid-term: at discharge (3mo)- Long term: 2y, 5y, 10y | VISA P | Improved at discharge (3 mo).No further changes in long term. |
| TEGNER SCORE | No significant changes | ||||||||
| SATISFACTION SCALE | 80% excellent at 3mo.No further changes in long term. | ||||||||
| Arias Buria30 | 2015 | RCT | Subacromial pain syndrome (Supraspinatus tendon) | 36 (9/27)Non-athletes | IG: PNEn= 17 | CG: Eccentric exercisesn= 19 | - Pre- Short-term: 2w.- Mid-term: 5w.- Long-term: n/a | PAIN | IG>CG in short & mid term |
| DASH | IG>CG in short & mid term | ||||||||
| Moreno31 | 2016 | RCT | Shoulder pain (Infraspinatus muscle & tendon) | 40 (20/20)Non-athletes | IG1: PNE tendon & trigger pointn= 10 | CG: No interventionn= 10 | - Pre- Short-term: 1w, 2w, 3w.- Mid-term: n/a- Long-term: n/a | PAIN | IG1>IG2>IG3>CG |
| IG2: PNE trigger pointn= 10 | ROM (shoulder ABD, IR, ER) | IG1>IG2=IG3>CG | |||||||
| IG3: PNE tendonn= 10 | TENDON STRUCTURE (US) | IG 1= IG 2= CG at mid and long term | |||||||
| Abat46 | 2016 | RCT | Patellar tendinopathy (Proximal patellar tendon) | 64 (51/13)“Athletically active subjects” | IG: PNE + Eccentric exercisesn= 32 | CG: Electrotherapy + Eccentric exercisesn= 32 | - Pre- Short-term: n/a- Mid-term: 2 mo.- Long-term: n/a | VISA P | IG=CG in mid-term |
| Moreno24 | 2016 | CASE SERIES | Rectus abdominis- related groin pain (Rectus abdominis tendon) | 8 (8/0)Athletes (professional football players) | IG: PNEn= 8 | N/A | - Pre- Short-term: 1d, 1w, 1 mo.- Mid-term: n/a.- Long-term: 6 mo. | PAIN | Improved at short and long term (1w, 1mo, 6mo) |
| FUNCTIONAL SCALE (PSFS) | Improved at short and long term (1w, 1mo, 6mo) | ||||||||
| Garcia Naranjo26 | 2017 | RCT | Whiplash syndrome (Levator scapulae muscle) | 100 (36/64)Non-athletes | IG: PNEn= 50 | CG: Electrotherapy + Manual therapy + Stretchingn= 50 | - Pre- Short-term: n/a- Mid-term: 5w.- Long-term: n/a | PAIN | IG=CG |
| NECK FUNCTION SCALE (npnq) | IG=CG | ||||||||
| PRESSURE PAIN | IG>CG in short term (5w) | ||||||||
| Moreno25 | 2017 | RCT | Adductor longus enthesopathy(Adductor longus tendon enthesis) | 24 (24/0)Athletes (non-professional football players) | IG: PNE + Eccentric exercisesn= 11 | CG: Eccentric exercisesn= 13 | - Pre- Short-term: at dicharge- Mid-term: 2 mo.- Long term: 4 mo, 6 mo. | PAIN PalpationPAIN contraction | IG>CG at 2 & 4 mo. IG=CG at 6 mo.IG>CG in mid and long term. |
| FUNCTIONAL SCALE (PSFS) | IG=CG at short, mid and long term | ||||||||
| De Miguel Valtierra29 | 2018 | RCT | Subracromial pain syndrome(Supraspinatus tendon) | 50 (23/27)Non-athletes | IG: PNE + Manual Therapy + Active Exercisesn= 25 | CG: Manual Therapy + Active Exercisesn= 25 | - Pre- Short-term: at dicharge- Mid-term: 3 mo.- Long term: 6 mo. | Pain | IG>CG in mid and long term (3 & 6mo) |
| DASH | IG=CG | ||||||||
| FUNCTIONAL QUESTIONNAIRE (SPADI) | IG>CG in mid and long term (3 & 6mo) | ||||||||
| PRESSURE PAIN THRESHOLD | IG=CG | ||||||||
| Self-rated improvement (Global Rating of Change- GROC) | IG>CG in mid and long term (3 & 6mo) | ||||||||
| Fernandez Rodriguez47 | 2018 | RCT | Plantar heel pain(Plantar fascia) | 67 (25/42)Non-athletes | IG: PNEn= 38 | CG: Placebo injectionn= 29 | - Pre- Short-term: 1w.- Mid-term: 12w.- Long term: 24w. | PAIN | IG>CG in short and long term (1w, 12w, 24w) |
| FUNCTIONAL QUESTIONNAIRE (FAAM) | IG>CG in short, mid and long term (1w, 12w, 24w) | ||||||||
| Structural change (Fascia thickness) | IG>CG Not clinically relevant | ||||||||
| Iborra-Marcos48 | 2018 | RETROSPECTIVE COMPARATIVE | Plantar fasciosis(Plantar fascia) | 64 (35/29)Non-athletes | IG: PNEn= 32 | CG: Corticosteroid injectionn= 32 | - Pre- Short-term: n/a.- Mid-term: 3 mo.- Long term: 6 mo, 12 mo. | PAIN | IG=CG |
| FUNCTIONAL QUESTIONNAIRE (FADI) | CG>IG in mid and long term (3mo, 6mo, 12 mo) | ||||||||
| Structural change (Fascia thickness) | IG=CG | ||||||||
| Lopez-Martos27 | 2018 | RCT | Temporomandubular myofascial pain (Lateral pterigoid muscle) | 60 (8/52)Non-athletes | IG1: PNEn= 20 | CG1: Deep dry needlingn= 20CG2: Sham needlingn= 20 | - Pre- Short-term: 4w.- Mid-term: 6w, 10w.- Long term: n/a. | PAIN AT RESTPAIN AT MASTICATION | IG1>CG1>CG2 at 4w and 6wIG1=CG1>CG2 at 10wIG1=CG1>CG2 at short and mid term |
| ROM (max interincisal opening) | IG1>CG1>CG2 at short and mid term | ||||||||
| FUNCTIONAL QUESTIONNAIRE (TMJ) | IG1=CG1>CG2 at short and mid term | ||||||||
| Al Boloushi49 | 2020 | RCT | Plantar heel pain (Calf and intrinsic foot muscles trigger points)) | 102 (30/72) Non-athletes | IG: PNE + stretching(n=51) | CG: Dry needling + stretching(n=51) | - Pre- Short-term: 4w- Mid-term: 8w, 12w- Long term: 26w, 52w | FUNCTIONAL QUESTIONNAIRE (Foot health Status Questionnaire) | IG=CG at short, mid and long term |
| PAIN | CG>IG at short termCG= | ||||||||
| Quality of life questionnaire (EQ-5D-5L) | IG=CG at short, mid and long term (26w)IG>CG at long term (52w) | ||||||||
| Rodriguez-Huguet40 | 2020 | RCT | Supraspinatus tendinopathy (Supraspinatus tendon) | 36 (27/9)Non-athletes | IG: PNE + eccentric exercise(n=18) | CG: Dry needling + eccentric exercises(n=18) | - Pre- Short-term: 1 mo.- Mid-term: n/a- Long term: 1y | PAIN | IG>CG at short and long term (close to minimal detectable change) |
| TRIGGER POINT PRESSURE PAIN THRESHOLD | IG>CG at short and long term(close to minimal detectable change) | ||||||||
| ROM | IG>CG at short and long term (close to minimal detectable change) | ||||||||
| Rodriguez-Huguet13 | 2020 | RCT | Epicondylalgia (Lateral epicondyle tendon) | 32 (20/12)Non-athletes | IG: PNE + eccentric exercise(n=16) | CG: Dry needling + eccentric exercises(n=16) | - Pre- Short-term: post, 1 mo,- Mid-term: 3 mo.- Long term: n/a | PAIN | IG>CG at short term (post, 1 mo and 3 mo) |
| Pressure pain threshold | IG=CG post-treatmentIG>CG at 1 mo and 3 mo | ||||||||
| ROM | IG>CG at short term (post, 1 mo and 3 mo) for FLEXIONIG=CG at short term for EXTENSION, SUPINATION & PRONATION. | ||||||||
| QUALITY OF LIFE QUESTIONNAIRE (SF-12) | IG=CG | ||||||||
| Valera-Garrido17 | 2020 | Case series | Rectus femoris Grade 2 injury (Rectus femoris | 13 (13/0)Professional football players | IG: PNE + Rehab and reconditioning program(n=13) | N/A | - Pre- Short-term: 7d, 14d (US)- Mid-term: 8w, 12w.- Long term: n/a | Return to train (RTT)Return to play (RTP) | RTT= 15.62 ± 1.80 daysRTP= 20.15 ± 2.79 days |
| MUSCLE STRUCTURE (US) | Improved tissue structure at 7 and 14d | ||||||||
| PERFORMANCE VARIABLES (GPS Match) | Similar performance compared to pre-injury | ||||||||
| Calderon Diez20 | 2020 | Case series | Chronic Achilles tendinopathy (achilles tendon) | 39 (33/6)Non-athletes | IG: PNE + eccentric exercises(n=39) | N/A | - Pre- Short-term: n/a- Mid-term: 3 mo- Long term: n/a | PAIN | Improved at mid term (3 mo) |
| FUNCTIONAL SCALE (FADI) | Improved at mid term (3 mo) | ||||||||
| VISA-A | Improved at mid term (3 mo) | ||||||||
| De la Cruz21 | 2020 | RCT | Chronic soleus injury (Soleus central tendon) | 30 (3/27)Ballet dancers | IG 1: PNE(n=10)IG 2: PNE + eccentric exercises(n=10) | CG: Eccentric exercises(n=10) | - Pre- Short-term: post-treatment- Mid-term: n/a- Long term: n/a | PAIN | IG1=IG2>CG |
| ROM (Ankle Dorsiflexion) | IG1=IG2=CG (Higher % of changes in IG2) | ||||||||
| MUSCLE ENDURANCE TESTS | IG1=IG2=CG (Higher % of changes in IG2) | ||||||||
| FUNCTIONAL SCALE (Dance Functional Outcome Survey- DFOS) | IG1=IG2=CG (Higher % of changes in IG2) | ||||||||
| PERCEPTION OF IMPROVEMENT (Minimal Clinically important difference MCID) | IG1=IG2>CG | ||||||||
| Lopez-Royo50 | 2021 | RCT | Patellar tendinopathy (patellar tendon) | 48 (42/6)100% Nonelite athletes | IG 1: PNE + eccentric exercises(n=16) | CG1: Dry needling + eccentric exercises(n=16)CG2: Sham needling + eccentric exercises(n=16) | - Pre- Short-term: n/a- Mid-term: 10 w- Long term: 22 w | PAIN | IG 1= CG 1= CG 2 at mid and long term |
| VISA-P | IG 1= CG 1= CG 2 at mid and long term | ||||||||
| QUALITY OF LIFE QUESTIONNAIRE (SF-36) | IG 1= CG 1= CG 2 at long termIG 1> IG 2> CG at mid term | ||||||||
| TENDON STRUCTURE (US) | IG 1= CG 1= CG 2 at mid and long term |
Pain was the most common primary outcome measure (76% of the studies), using subjective patient-reported pain scales like visual analogue scale or numeric rating score. Injury-related function was also reported as a primary outcome measure in most studies using validated questionnaires for each specific injury type: VISA-P for patellar tendon,10,44–46,50 VISA-A for achilles tendon,20 DASH and SPADI for upper limbs injuries,12,30,29 NPNQ for whiplash syndrome,26 temporomandibular joint questionnaire,27 and Foot Health Status,49 FADI20,48 and FAAM49 for foot and ankle pathologies. Changes in tissue structure were measured in 7 studies,50,17,48,47,31,12,44 mostly by monitoring ultrasound changes in tissue thickness, echogenicity, and neovascularization.
Secondary outcome measures included range of motion,31,27,13,40 general function and activity scales (Tegner Activity Scale,10,45 Patient Specific Functional Scale (PSFS),24,25 specific activity scales (Dance Functional outcome survey DFOS, [21]), and Quality of life questionnaires (EQ-5L-DL [49], SF-36 [51] and SF-12 [13,40]).
Performance-related measures like muscle endurance tests and football performance variables were reported in two studies with athletes17,21 and patient-reported satisfaction and perception of success were also reported by several authors10,12,45,21,29
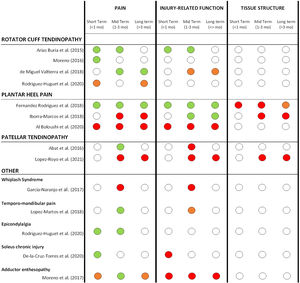
Percutaneous needle electrolysis effects for different pathologiesDetails about the reported effects of PNE on all reported outcome measures can be found in Table 3. A summary of short, mid and long-term effects of PNE on pain, injury-related function and tissue structure only for comparative studies (grouped by pathology types) is presented in Fig. 2.
Summary of short, mid and long-term effects of PNE on pain, injury-related function and tissue structure for all comparative studies (grouped by pathology types). Color code: GREEN= significant positive effect of PNE compared to other treatment modalities or control groups. ORANGE= limited or unclear positive effect of PNE (not in all reported measures or non-clinically relevant). RED: no superior effect of PNE compared to other treatment modalities or control groups. WHITE: not reported.
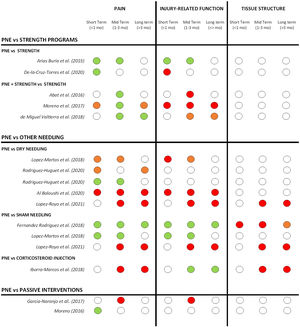
For most comparative studies included in this review, PNE was compared with 3 main treatment approaches: strength programs, other needle interventions (dry needling, sham needling, or corticosteroid injections) and passive modalities.
A summary of the effects of PNE on pain, injury-related function and tissue structure compared to other treatment modalities at short, mid and long term is shown in Fig. 3.
Summary of short, mid and long-term effects of PNE on pain, injury-related function and tissue structure for all comparative studies (grouped by the treatment modalities used in the comparison groups). Studies with more multiple comparison groups are displayed for each one of them. Color code: GREEN= significant positive effect of PNE compared to other treatment modalities. ORANGE= limited or unclear positive effect of PNE (not in all reported measures or non-clinically relevant). RED: no superior effect of PNE compared to other treatment modalities. WHITE: not reported.
The aim of this review was to explore the status of the scientific evidence for the use of percutaneous needle electrolysis in musculoskeletal injuries, as well as clarifying its indications and reported effects.
Overall, there was a high heterogeneity of clinical study types, a wide range of musculoskeletal pathologies, and multiple protocols and approaches. Positive effects on pain and injury-related function were documented by several studies at various time-points. No improvements in tissue structure could be attributed to the treatment with PNE. However, these findings should be interpreted with caution based on the results of this review due to several reasons:
Research qualityPercutaneous needle electrolysis is a relatively novel technique, so high-quality research studies are still scarce and delayed compared to its use in clinical practice. The oldest clinical study found in this review is a case series published by Valera et al. in 2010,44 and the first comparative study is from Arias-Buria et al in 2015.30 Research quality is also limited: more than 60% of all clinical studies included in this review were at moderate or high risk of bias based on the MINORS score. Inadequate reporting about blinding protocols, lack of prospective calculation of study size, and inadequate design of control groups were identified as the most common risks of bias.
Regarding the blinding procedures for participants and therapists, this has already been identified as one of the main challenges for clinical trials comparing different therapeutic needling procedures,51 and there is a lack of consensus on blinding protocols potentially affecting results for these kinds of studies.
Comparison with other treatment modalitiesPNE vs other needle interventionsWhen compared with other treatment modalities, most studies compared PNE with other needle interventions like dry needling, corticosteroid injections or placebo needling.
It's important to highlight that dry needling was often applied in different manners compared to PNE: Lopez-Royo et al50 applied both PNE and dry needling directly over the injured area (patellar tendon) following the same protocol blinded to the patient; Lopez Martos et al.27 and Al Boloushi et al49 applied both PNE and dry needling in the muscle trigger points; and Rodriguez Huget13,40 applied PNE at the injured tendon and dry needling at the trigger points of the related muscles. For this reason, the comparison between PNE and dry needling must be interpreted with caution since they are often not targeting the same tissue structures and may cause different therapeutical responses. One author31 compared the effects of PNE on different areas of the supraspinatus (tendon, muscle trigger points or combined), finding better results for the combined group.
Three studies have compared PNE with placebo needling27,47,50 with opposing outcomes. Lopez-Royo et al50 reported no differences between PNE and placebo needling. In this study, a sham needle was placed on the skin simulating the treatment procedure with the electrical current device turned off next to them and the therapist insisting on the whole therapeutic experience beyond the mere tactile stimulation. This is the only study in which blinding was assessed post-intervention and most patients included in the sham needle group could not guess what group they belonged to, while all patients included in the intervention group could guess they were receiving PNE. However, they did not show superior outcomes compared to the sham needling group. Two other studies showed superior outcomes with PNE compared to sham needling. Lopez-Martos et al27 designed a double-blinded study (only the principal investigator was blinded, not the therapist) applying a superficial skin puncture with the plastic cover in the sham needling group, and with the electrotherapy device simulating the same noise as with the PNE application. Finally, Fernandez-Rodriguez et al47 blinded both the patient and the therapist, performing the same needle intervention in both groups but turning off the ultrasonographic monitor so that the therapist could not see if an electrical current was being applied or not.
One study compared PNE with corticosteroid injection for plantar heel pain (Iborra-Marcos [48]), finding better outcomes in the PNE group for injury-related function, but no differences in pain or tissue structure in mid and long term.
PNE vs active exerciseOnly eight studies used PNE in isolation for the intervention group. The vast majority combined PNE with different types of active exercise interventions; eccentric exercises,44,12,10,46,25,40,13,21,20,50 isoinertial strength protocols,45 stretching,12,49 rehab and reconditioning program17 or manual therapy combined with active physical exercise.29
Five studies compared PNE with active interventions. PNE showed better improvements in pain when used in combination or compared with strength only, especially in the mid-term (1-3 months), but this did not reflect in a better injury-related function for most studies except Arias-Buria.30 It's important to remark that the studies that compared PNE with strength interventions were applied in different pathologies and body regions for both upper and lower limbs: shoulder rotator cuff tendinopathy,29,30 patellar tendinopathy,46 soleus chronic injury21 and adductor enthesopathy.25 Strength protocols also differed between studies: twice a day, 3 sets of 12 repetitions for 4 to 5 weeks29,30; 3 sets of 15 repetitions (frequency not reported)46; 3 sets of 15 repetitions, 4 days a week for 4 weeks after dancing classes21; or a progression of exercises from isometric to eccentric to isoinertial eccentric.25
Since most studies targeting tendons combined PNE with different kinds of active exercise interventions (which remains the gold standard for tendinopathy management52,53), it's difficult to attribute all improvements to PNE, but it may be considered as an adjunct for pain management in some cases.
Application protocols and indicationsOne of the major limitations observed in the studies is the difference between application protocols. For most studies, the galvanic current was used at an intensity ranging from 1.5 to 6mA in bouts of 3 to 5 seconds and repeated 1 to 5 times per session. However, four RCTs30,29,13,40 used a completely different set of parameters: lower intensity (0.35mA) and longer exposure (1.2 to 1.5min). While all studies using this protocol were applied in shoulder and epicondylalgia, there is no justification about when one protocol or the other is indicated, so it's unclear if the results of both protocols should be compared under the same conditions.
Most studies (n=15) applied PNE once a week, but the total duration of the treatment differed significantly; while some studies had a fixed protocol duration (ranging from 2 to 8 weeks), others were based on the clinical progression of symptoms,44,45,10,46,24,48,25 which ranged from 4 days31 to 10 weeks.10,45,48
PNE was applied in a wide range of injury types with differing pathophysiology: upper body and lower body tendinopathies, enthesopathies, acute and chronic muscle injuries, temporomandibular myofascial pain, subacromial pain syndrome, plantar heel pain and whiplash syndrome; therefore, a specific treatment indication and timing for application is still unclear, so comparisons must be made with caution.
PNE treatment effectsPainA recent systematic review by Gomez-Chiguano et al (2021),54 found moderate evidence of a positive effect of PNE on pain and pain-related disability in short, mid and long term for musculoskeletal conditions. However, the current body of evidence is scarce and difficult to interpret due to the differences in the way pain is measured across studies.
Different pain domains have been used, alone or in combination: maximum pain, minimal pain, average pain, pain at rest, pain on palpation, pain with muscle contraction, pain at work, pain during daily activities, pain during sport activities or pain with movement are some examples used in the studies included in this review. Three studies26,12,29 also measured the pressure pain threshold at the affected area using an algometer. Since different approaches were used to measure pain changes, and pain is subjective, it's difficult to establish clear comparisons between studies.
However, the best overall effects of PNE in our review were found for the pain domain, especially in shoulder-related tendinopathies at different time points.30,31,29,40 For other pathologies like plantar heel pain the results were conflicting: Fernandez-Rodriguez et al.47 reported a larger pain decrease with PNE compared to a placebo puncture in short, mid, and long term, while Al Boloushi et al49 and Iborra-Marcos48 didn't find any difference when PNE was compared to corticosteroid injection or dry needling. For patellar tendinopathy the results were conflicting as well; Abat46 reported superior effects of PNE combined with strength compared with strength only in the mid-term, but Lopez-Royo et al.50 reported PNE was not superior to dry needling or sham needling in mid and long term. For other pathologies, positive outcomes in terms of pain were found for temporomandibular joint dysfunction, epicondylalgia and soleus chronic injury, while conflicting or not superior outcomes were found for whiplash syndrome and adductor enthesopathy. Overall, better results on pain were found in short and mid-term (up to 3 months) compared to long term (more than 3 months).
Injury-related functionImprovements in injury-related function with PNE were reported for subacromial pain by Arias-Buria et al.,30 compared to strength training at short and mid-term, and for plantar heel pain compared to sham needling47 and corticosteroid injections48 at different time points. However, some studies reported PNE did not have a superior effect on injury-related function compared to other treatment modalities or control groups for plantar heel pain,49 patellar tendinopathy,46,50 whiplash syndrome,26 soleus chronic injuries21 and adductor enthesopathy.25 For non-comparative studies there are also conflicting findings, with no improvements reported for patellar tendinopathy10,45 and positive effects found in achilles tendinopathy20 and groin pain function.24
However, functional scales may not necessarily reflect specific injury function in the same manner for different injury types. For example, VISA-P and VISA-A (used in all patellar and achilles tendon studies included in this review) are the gold standard for tendinopathy, while DASH (Disability of arm, shoulder, and hand) and SPADI (Shoulder Pain and Disability Index) (used in some rotator cuff tendinopathy and epicondylalgia studies) are not pathology-specific but general upper body functional scales.
As a summary, while there is evidence of a positive effect on PNE on pain, there is inconclusive evidence about the effectiveness of PNE for improving injury-related function.
Tissue structureStructural changes were measured by ultrasound in four comparative studies in patellar tendon,44 lateral epicondylalgia12 and plantar fasciitis,47,48 but no positive effects after PNE treatment were found.
Only Fernandez-Rodriguez et al47 documented a statistically significant decrease in plantar fascia thickness compared to the control group at 6 months. However, the authors acknowledged that a 0.3mm decrease may lack true relevance since changes below 0.7mm can be attributed to the intra-rater typical error.55 Other case series studies have reported improvements in tissue structure in patellar tendon12 and rectus femoris,17 but it's difficult to attribute those effects to PNE in the absence of a control group.
We can conclude that there isn't enough evidence to support the effect of PNE on promoting structural tissue changes.
OthersTwo RCT studies measured changes in range of motion for shoulder,31 and temporo-mandibular joint (TMJ)27; two studies assessed patient-reported perception of improvement or success,12,29 and two reported the patient satisfaction with the technique.10,45
For range of motion, PNE seems to improve shoulder mobility when applied in the infraspinatus muscle and tendon combined,31 and the same was observed for the TMJ when applied at the lateral pterygoid muscle.27 However, the small sample size of these studies and the magnitude of changes observed should prevent from extrapolating these effects to other pathologies and applications of the technique.
Between 79 and 88% of the subjects reported high satisfaction with the technique beyond 3 months,10,45 however no comparison can be made as there was no control group for these studies. A similar thing happens with the perception of success, which is reported as excellent by all patients in a case series study12 without a control group. Regarding the perception of improvement, more patients reported moderate to large improvements at 3 and 6 months in the PNE group compared with a control group in a shoulder RCT.29
Since there are many methodological limitations related to the use of subjective measures in low quality studies with small sample sizes, no clear conclusions can be extracted about the general effects of PNE in the patient perception of improvement and satisfaction.
ConclusionIn summary, the current body of scientific evidence shows that PNE is being used in a wide range of pathologies (predominantly tendon-related), but a consensus about treatment indications, dosage and application protocols is still lacking.
PNE seems to have positive effects on pain in short and mid-term when compared with active exercise interventions alone and sham needling, but the comparison with corticosteroid injections, dry needling and passive interventions is inconclusive. There is limited evidence for PNE to improve injury-related function and no evidence of tissue structure improvement when PNE is applied.
More high-quality studies are needed to define PNE indications, recommended parameters and potential effects on different pathology types and stages.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.