Since the 1980s the role of tryptophan in modulating fatigue has been the subject of debate. According to some models, tryptophan delays the onset of fatigue. Conversely, in other models, this amino acid is implicated in generating fatigue. This study attempts to shed some light on the debate.
MethodTen elite (non-professional) road racing cyclists took part in a double-blind study employing paired data under standardised dietary and training conditions. Each volunteer did two trials, one with tryptophan supplementation (TRP) and the other with placebo (PLB). Various body fuel, plasma amino acid and renal function parameters were analysed in relation to fatigue data obtained using the Borg test. Blood pressure, pupil size and haematocrit values were also monitored. The parameters obtained were used to examine the principal components of fatigue in said test.
ObjectiveTo confirm that, under test conditions, oral L-tryptophan supplementation has a favourable influence on fatigue perception during exercise.
ResultsThe principal components of fatigue, as reflected by the Borg test in this study, fit the following model: Perception of fatigue attained=8.406050586+(0.126275247*% load intensity)−(0.031521537*cholesterol mg/dl)+(0.051002322*HDL cholesterol mg/dl)−(0.017130681*tryptophan mmol/ml)−(0.004545865*glycinemmol/ml)+(0.082894085*methioninemmol/ml). (Multiple correlation coefficient=0.90; coefficient of determination R2=0.82; adjusted R2=0.81; standard error: 1.82; observations: 75).
Renal functionUrea: (TRP: 45.6±6.5mg/dl vs. PLB: 44.1±5.7; p<0.4); creatinine: (TRP: 90.4±12.0 vs. PLB: 88.4±11.8mg/dl; p<0.9); resting pupil size: (TRP: 0.38±0.07AU vs. PLB: 0.35±0.08AU; p<0.01); haematocrit: (TRP: 45.3%±1.5% vs. PLB: 46.1%±2.6%; p<0.40).
ConclusionsHigher tryptophan levels reduced the increased subjective perception of fatigue (SPF). In the component analysis the impact of tryptophan was estimated as 4% in our model. Tryptophan supplementation did not affect renal function. Tryptophan supplementation altered pupil size. At the dose used, tryptophan supplementation proved effective for the first 2h following the last intake of the tryptophan supplement.
Desde los años 80, el papel del triptófano para modular la fatiga ha sido objeto de debate. Con arreglo a algunos modelos, el triptófano demora el inicio de la fatiga. Por el contrario, en otros modelos, dicho aminoácido está implicado en la generación de fatiga. Este estudio trata de arrojar cierta luz al debate.
MétodoDiez ciclistas de carretera de élite (no profesionales) tomaron parte en un estudio doble ciego utilizando datos pareados en condiciones dietéticas y de entrenamiento. Cada voluntario realizó dos pruebas: una con suplemento de triptófano (TRP) y otra con placebo (PLB). Se analizaron diversos parámetros en cuanto a energéticos corporales, aminoácidos en plasma y función renal, con relación a los datos sobre fatiga obtenidos mediante la prueba de Borg. También se supervisaron los valores de presión arterial, tamaño de la pupila y hematocrito. Los parámetros obtenidos se utilizaron para examinar los componentes principales de la fatiga en dicha prueba.
ObjetivoConfirmar que, en condiciones de prueba, el suplemento oral de L-triptófano influye favorablemente en la percepción de la fatiga durante el ejercicio.
ResultadosLos componentes principales de la fatiga, reflejados en este estudio mediante la prueba de Borg, se ajustaron al modelo siguiente: Percepción de fatiga obtenida=8,406050586+(0,126275247*% intensidad de carga)−(0,031521537*colesterolmg/dl)+(0,051002322*HDL colesterol mg/dl)−(0,017130681*triptófanommol/ml)−(0,004545865*glicina mmol/ml)+(0,082894085*metionina mmol/ml). (Coeficiente de correlación múltiple=0,9; coeficiente de determinación R2=0,82; R2 ajustado=0,81; error estándar: 1,82; observaciones: 75).
Función renalUrea: (TRP: 45,6±6,5mg/dl vs PLB: 44,1±5,7; p<0,4); creatinina: (TRP: 90,4±12 vs. PLB: 88,4±11,8mg/dl; p<0,9); tamaño de la pupila en reposo: (TRP: 0,38±0,07 AU vs. PLB: 0,35±0,08 AU; p<0,01); hematocrito: (TRP: 45,3%±1,5% vs. PLB: 46,1%±2,6%; p<0,4).
ConclusionesLos niveles superiores de triptófano redujeron el incremento de percepción subjetiva de la fatiga (SPF). En el análisis de componente, el impacto del triptófano se calculó como un 4% en nuestro modelo. El suplemento de triptófano no afectó a la función renal, pero sí alteró el tamaño de la pupila. A la dosis utilizada, dicho suplemento de triptófano resultó ser efectivo durante las dos primeras horas siguientes a la última ingesta del mismo.
Javierre et al. state that: “Strenuous exercise may be associated with discomfort and pain that may limit physical performance. Failure to generate or maintain the desired or expected force may occur as a result of impairment in one or more of the steps from the cerebral cortex to the muscle fibre.”1
Thus, in many cases, physical exercise is terminated not because of muscle fatigue, but due to neuronal insufficiency entailed in exercise 2,3. The serotonergic system participates in the modulation of sensory information, apparently reducing nociception and irrelevant information through its effects on the cell bodies of neurons located in the dorsal horn of the spinal cord 4. The bioavailability of 5-hydroxytryptamine (5-HT) (serotonin) depends on the uptake of L-tryptophan. In turn, the uptake of this amino acid is dependent on its plasma concentration and on its ratio to the sum of the other large neutral amino acids 5,6,7,8,9. Although some studies 10,11 suggest a decrease in performance, in prolonged exercise, with increased 5-HT, others do not 12. A previous study showed that moderate supplementation with L-tryptophan (600mg/day) substantially improved endurance time in a high-intensity test in a group of 12 healthy sportsmen, in the laboratory.13
For further in-depth analysis of this issue, an experiment was conducted with cyclists in 1996. One aim was to support previous findings and overcome statistical limitations by using an experimental design better suited to the observation of tryptophan supplementation. Another aim was to standardise to a high degree the physical demands made on the volunteers being tested. Experimenting on cyclists, under laboratory conditions, made it possible to use analytical procedures such as high-performance liquid chromatography (HPLC). These techniques provided additional information about tryptophan and other amino acids, as well as various biochemical parameters, when plasma tryptophan levels were altered by means of dietary manipulations.
Hypothesis and objectivesHypothesis- 1.
Tryptophan plays a role in modulating the sensations that accompany physical exhaustion. If cyclists are given tryptophan supplements, a delay will be observed in the onset of sensations that accompany fatigue. This will be reflected in measurements of subjects’ subjective perception of fatigue.
- 2.
Assuming that tryptophan intake does not alter the proportions or bioavailability of body fuels, the process of exhaustion induced by prolonged physical exercise under test conditions should be apparent from changes in blood levels of body fuels, and will be unaffected by tryptophan supplementation.
- 3.
Supplementing the diet with tryptophan does not affect renal function.
- 1.
To determine the significance of differences in fatigue perception with or without tryptophan supplementation under laboratory conditions.
- 2.
To determine the relationship between tryptophan supplementation and test subjects’ lipid profile, and the influence of blood lipid levels on altering fatigue perception.
- 3.
To study the influence of tryptophan supplementation on the relative proportion of plasma levels of branched-chain amino acids (BCAA).
- 4.
To study the influence of tryptophan supplementation on the transfer of plasma tryptophan through the blood/brain barrier. To measure the ratio of tryptophan to large neutral amino acids (trp/LNAA) and pupil size differences in the eyes as possible evidence of tryptophan crossing that barrier.
- 5.
To rule out any impact (negative or otherwise) of tryptophan supplementation on the kidneys.
- 6.
To study the impact of tryptophan supplementation on bioavailability of the following body fuels: glucose and triglycerides.
The study involved 10 volunteer élite cyclists, aged under-23, at the end of the 1996 season. They were all active at competition level. Subjects were regularly monitored by sports medicine practitioners and were in good health.
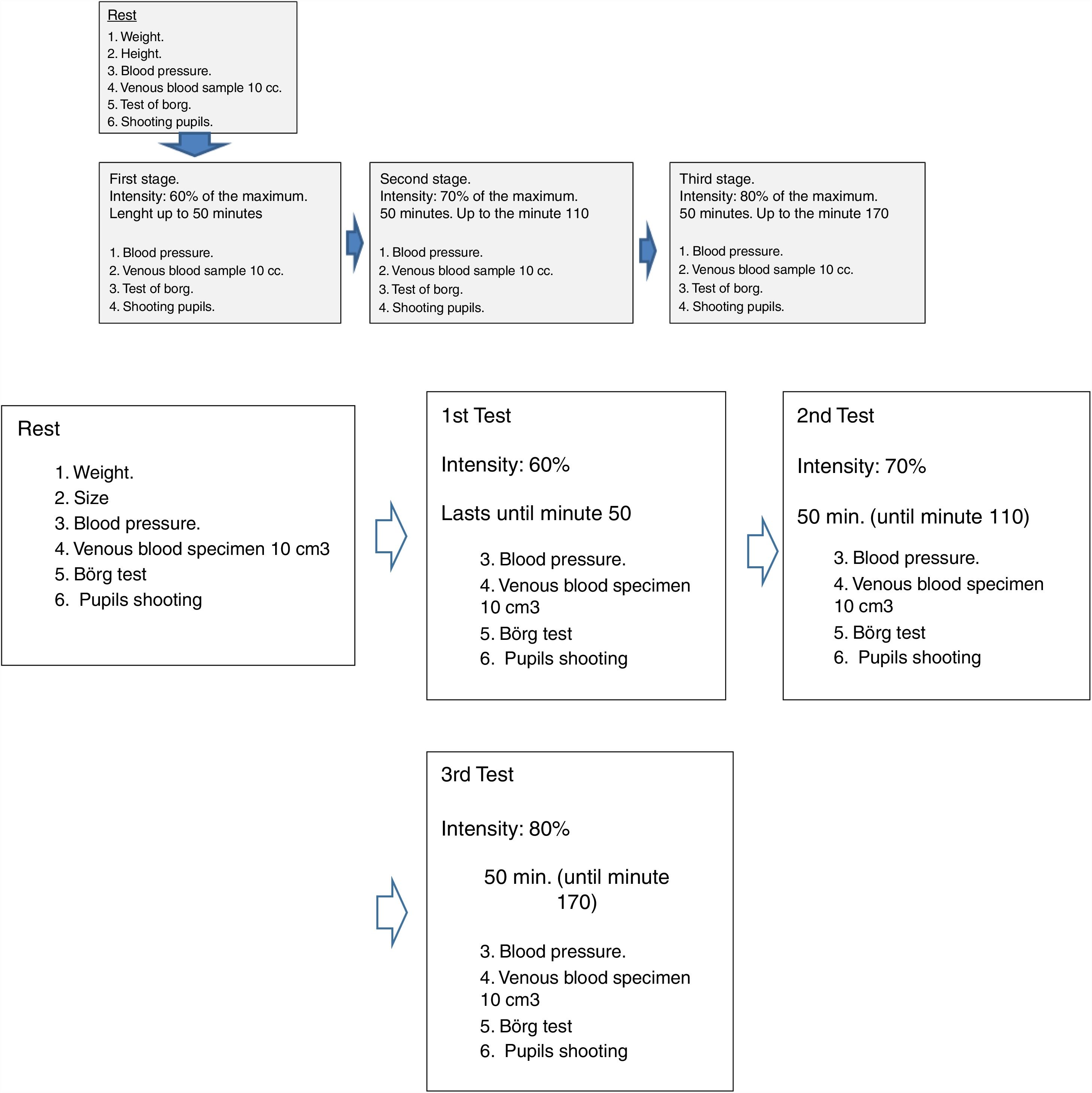
Data were collected in a mixed setting, i.e. with subjects’ own bicycles, but under double-blind laboratory conditions, with maximum standardisation in terms of physical performance. Trials took place in the laboratories of the University of Barcelona's Department of Human Physiology and Nutrition. Using their own bicycles and portable rollers, subjects performed two identical trials one week apart: three bouts of 50min of work, separated by 10min of recuperation between bouts. The three bouts were performed at intensities of 60%, 70% and 80% of each subject's current maximum heart rate. Subjects wore a heart rate monitor so that working heart rates could be recorded and, thus, enabling accurate adjustment of the heart rate pre-set for each subject in each bout. Subjects underwent trials lasting approximately 3h. The rollers that were employed are routinely used by road racing cyclists for supplementary home training. The gear shifts used in each bout were noted, to be taken into consideration a week later, when the repeat trial took place.
The two trials were used to look for differences in fatigue perception obtained in two different situations:
- a)
With a low-tryptophan food for 24h prior to testing plus a supplementation with placebo 30min before the trial (10mg/body weight)
- b)
Low-tryptophan food for 24h prior to testing plus a supplementation with L-tryptophan 30min before the trial (10mg/body weight).
The order of the trials was random, so the first trial involved placebo supplementation in some subjects and tryptophan supplementation in the others. Although subjects had been informed of the nature of the experiment, they did not know when they were taking placebo or tryptophan.
Details of dietary regimenThe evening before the trial: Dinner consisting of a first course of pasta, rice or vegetables, followed by a second course of hot dogs, followed by fruit.
The morning of the testing day: Breakfast (on the day of the trial): As usual. Advised to limit milk intake to one medium glassful. Plenty of cereals, fruit, bread.
Advised to avoid: Fish, meat (except black pudding and frankfurters, which were permitted), sausages and ham (except mortadella, which was permitted). Peanuts, sunflower seeds and chocolate were also not permitted.
Supplementation with tryptophan or placebo: The corresponding products were dissolved in pineapple juice and provided in a brown bottle labelled with the time and a code to enable the nature of the solute to be identified later (10mg per kg body weight).
Dosage: dose 1: Wednesday at 5 pm/dose 2: Wednesday at 10 pm/dose 3: Thursday, 30min before the experiment began.
Data were collected as illustrated in the following diagram (Fig. 1).
The following parameters were measured in the blood samples obtained from the antecubital vein: Haematocrit value, lactic acid, glucose level, total cholesterol, HDL cholesterol, triglycerides, creatinine, uric acid, free fatty acids (in four subjects). In addition, the plasma concentration of de following amino acids were also measured by HPLC (glycine, valine, isoleucine, leucine, tyrosine, phenylalanine, methionine and tryptophan. The samples were prepared for chromatographic analysis in our laboratory, which was performed by the Scientific and Technical Department of the University of Barcelona, according to an officially approved procedure. That uses a cation exchange resin column.
Glucose, cholesterol, HDL cholesterol, triglycerides, creatinine. And uric acid were measured using fresh blood (within 4h of collection) with a Boehringer Mannheim Reflotron® analyser. Samples were stored at 2°C in a closed container.
Free fatty acids (FFA) in serum were determined quantitatively by the Wako Chemicals GmbH NEFA C in vitro enzymatic colorimetric method.
Haematocrit values were obtained by direct measurement after centrifugation of blood in capillary tubes.
Pupil size was measured in arbitrary units (AU), by filming the eyes with a Panasonic© VHS-C Movie Camera NV-S1 in the rest period between bouts, during the trials with placebo and tryptophan.
Statistical analysisQuantitative data were used to calculate the mean and standard deviation. The corresponding graphs were plotted separately for different work intensities and supplementation types. The principal components of fatigue (SPF) were analysed by multiple regression analysis, eliminating items successively until arriving at a formula containing items of statistical significance. The analysis was performed using only the statistical package included with Microsoft Excel 2010.
ResultsThe data and comparisons in Fig. 2 can be summarised as follows:
1. Thirty minutes after the third dose was taken, before the cycle ergometry trial began, the different test parameters measured showed no significant differences, except for relative pupil size (PLB x: 0.38±0.07AU vs. TRP x: 0.35±0.08AU; p<0.012).
2. At the end of the first stage (60% intensity) the increase in plasma tryptophan compared with the placebo group was clearly apparent: (PLB x: 33.73±17.11mmol/ml vs. TRP x: 93.26±26.16mmol/ml; p<0.00007). Differences were also observed in the concentrations of: isoleucine (ILE): (PLB x: 59.40±16.66mmol/ml vs. TRP x: 47.30±13.9mmol/ml; p<0.02) and tyrosine (TYR) (PLB x: 71.40±18.34mmol/ml vs. TRP x: 58.40±16.89mmol/ml; p<0.01) and in the ratios tryptophan/LNAA: (PLB x: 0.07±0.02 vs. TRP x: 0.21±0.05; p<0.00000) and tryptophan/5LNAA: (PLB x: 0.07±0.02 vs. TRP x: 0.22±0.05; p<0.00000).
3. There was still a clear difference in blood tryptophan levels, two hours after the third oral dose of L-tryptophan was taken after the second stage (70% intensity): (PLB x: 36.37±16.58mmol/ml vs. TRP x: 95.06±40.88mmol/ml; p<0.0012). There were also differences in the tryptophan/BCAA and tryptophan/LNAA ratios. These involve tryptophan, so it is not surprising that they were also affected.
4. After the third stage (80% intensity), there was a tendency for blood tryptophan levels to return to normal levels in the tryptophan supplementation group, but the differences were still significant. (Tryptophan: PLB x: 37.10±13.47mmol/ml vs. TRP x: 66.54±31.96mmol/ml; p<0.03). The tryptophan/BCAA and tryptophan/5LNAA ratios, in which tryptophan is involved, also showed significant differences. In the case of triglycerides, it should be noted that differences tending towards significance were seen in the third stage (80% intensity), with greater bioavailability of triglycerides in the blood when tryptophan supplementation took place: (Triglycerides: PLB x: 97.82±26.93mg/dl vs. TRP x: 123.83±47mg/dl; p<0.07).
5. Table 3 shows the differences in the various parameters at the end of the trial, compared with the beginning. Some variations were apparent according to whether the subject had received either placebo or tryptophan supplements. Glucose levels were significantly higher at the last trial compared with those at the beginning of de trial, in the TRP test group: (PLB: 93.63±12.29mg/dl vs. TRP: 101.79±11.47mg/dl; p<0.029). In regard to glucose, although it showed a clear downward trend at the end of the experiment, the differences were not significant Table 1. Table 2 shows that, at successive stages and exercise intensities, the volunteer cyclists displayed higher mean plasma glucose values when given tryptophan supplements. Fig. 3 shows that the tryptophan supplementation group had significantly higher final blood glucose levels. This could be interpreted in accordance with the observations made by Farris et al.14 (87). These authors found that standard exercise had less effect on muscle glycogen stores in horses given tryptophan supplements. Accordingly, the higher glucose figures in cyclists given tryptophan supplements might be due to lower consumption during exercise.
Evolution of plasma tryptophan levels from the start of the trial.
| Time (min) | Placebo | DS | Tryptophan | DS | p |
|---|---|---|---|---|---|
| 0 | 28.8 | 13.6 | 32.8 | 11.8 | <0.4 |
| 50 | 33.7 | 17.1 | 93.3 | 26.2 | <0.0001 |
| 110 | 36.4 | 16.6 | 95.1 | 40.9 | <0.0012 |
| 170 | 37.1 | 13.5 | 66.5 | 32 | <0.0302 |
Supplementation with placebo (placebo) vs. supplementation with Tryptophan. n=10 cyclists/double-blind/random assignment.
6. Cholesterol had fallen significantly in both situations by the exercise. In contrast, HDL cholesterol had risen significantly in the tryptophan supplementation group, as can be seen by comparing the final result against resting parameters (see Fig. 3).
Data on improved fatigue perception according to whether subjects had been treated with tryptophan or placebo were analysed by cross-tabulation and 2×2 contingency tables, because of their qualitative nature: Test for comparing two observed proportions in paired data sets (Domènech i Massons J.M. Bioestadística [Biostatistics]; Published by Herder, Barcelona. [1982] p. 204) (with the corresponding summary statistics under the same conditions as in Section 2.3): (Fig. 4).
7. Assessment of the impact on renal function: The behaviour of subjects’ blood creatinine levels does not suggest renal function overload, despite significant differences at the end of the trial in the tryptophan supplementation group, because levels remained within the normal range (normal values between 70 and 130mg/cl). Also, none of the subjects showed evidence of urea levels above the normal values (18–55mg/dl) (see Fig. 3).
Levels of tryptophan in blood samples collected at minutes 0, 50, 110 and 170 from the beginning of the trial with placebo or with tryptophan supplementation. Abbreviations: HDL, HDL cholesterol fraction; TRYP, L-Tryptophan; Pupil, relative size of the pupil; Fatigue, tip of fatigue perception (Borg's scale); GLN, Glycine; VAL, valine; ILE, isoleucine; LEU, leucine; TYR, Tyrosine; PHE, phenylalanine; MET, methionine; 3 (val, ile, leu), the sum of the value of the 3 branched chain amino acids (BCAA); 6 (v-i-l-t-p-m), sum of the values for the six amino acids: valine, isoleucine, leucine, tyrosine, phenylalanine and methionine; TRYP/BCAA, tryptophan quotient divided by branched amino acids; TRYP/5LNAA, ratio of the Tryptophan with relation to the neutral long chain amino acids.
8. Tryptophan supplementation altered the profile of other amino acids, as clearly seen in the case of valine, leucine and isoleucine. Although this was not significant (except for isoleucine at the end of the first 50min of exertion), there were significant differences compared with the resting state in the tryptophan supplementation group.
9. Analysis of the principal components associated to the perception of fatigue, by the Borg test, were based on the following components: workload, blood glucose, cholesterol, HDL cholesterol, tryptophan, glycine, valine, isoleucine, leucine, tyrosine, phenylalanine, methionine, branched-chain amino acids, sum of six amino acids, ratio of tryptophan to branched-chain amino acids (trp/BCAA) and ratio of tryptophan to long-chain neutral amino acids (trp/5LNAA). The principal components of fatigue, as reflected by the Borg test in this study, fit the following model.
10. Perception of fatigue attained=8.406050586+(0.126275247*% load intensity)−(0.031521537*cholesterol mg/dl)+(0.051002322*HDL cholesterol mg/dl)−(0.017130681*tryptophan mmol/ml)−(0.004545865*glycine mmol/ml)+(0.082894085*methionine mmol/ml).
(Multiple correlation coefficient=0.90; coefficient of determination R2=0.82; adjusted R2=0.81; standard error: 1.82; observations: 75).
The coefficients were normalised in percentage form (Table 4). In total, they added up to an adjusted R2 of 0.81. Accordingly, the involvement of tryptophan in this case, under these study conditions, was 4%, against the onset of fatigue. The impact of supplementation with tryptophan, in the present study, is a point on the Borg scale.
11. Time course of haematocrit: see Table 5.
Discussion of the resultsThe results of this study, performed on elite (non-professional) road cyclists, show that 85% of the subjects, under laboratory conditions, had less perception of fatigue when given tryptophan supplements, at a dosage of 10mg per kg body weight at diner time of the previous day, at breakfast time of the experimental day and 30min before the beginning of the trial, in a 3-h, double-blind, crossover trial of progressive intensity.
These results are consistent with those observed by Segura and Ventura13 and Javierre et al.1 In contrast, they contradict the findings of Strome et al.15 In humans, and Farris et al.14 in horses. The latest authors observed a lower work capacity in horses given tryptophan supplements, while muscle glycogen stores were higher after exercise. It is obviously impossible to discuss subjective perception of fatigue in horses.
Chromatographic analysis of plasma ingested amino acids in the group of cyclists showed that, the tryptophan 30min before the experimental trial, it was rapidly absorbed and within half an hour of its ingestion, plasma tryptophan levels were 4-fold higher, and 3h later those levels were still 3 times higher than normal. This occurred when the cyclists had taken tryptophan supplements, as shown in Table 2 and Fig. 1.
After 30min of its ingestion there was also evidence that the transference of tryptophan across the blood/brain barrier was increased. This was apparent from the TRYP/LCNA ratio described by Fernstron and Faller16 in mice and discussed by Gillman et al.17 in humans. In the cyclist study, this ratio increased 4-fold when subjects had taken tryptophan, and this increase was still double after 3h (Fig. 3).
The model proposed by Fernstrom,18 based on the existence of a carrier at the blood/brain barrier, should be reviewed. This carrier enables tryptophan to enter the brain in competition with LNAAs. Our study results do not agree, because plasma levels of LNAAs restricted from entering the brain because of a rise in plasma tryptophan should stay the same as with placebo or slightly higher. That was not the case. Instead, when tryptophan was administered, plasma levels of LNAAs (tyrosine, phenylalanine, leucine, isoleucine and valine) clearly decreased in all cases. In the case of isoleucine, this was statistically significant (Table 3). This raises new questions about the trp/LNAA ratio as an indicator of tryptophan passage into the brain. It might mean that competition between tryptophan and LNAAs exists before the blood/brain barrier too.
The “p” statistics of different parameters analysed at rest, compared with the values at 80% at maximum intensity, discriminating the statistical significance “p” placebo vs. “p” tryptophan.
| Resting vs. 80% intensity | ||
|---|---|---|
| Parameter | Placebo | Tryptophan |
| p | p | |
| Glucose | <0.5026 | <0.0293 |
| Cholesterol | <0.0191 | <0.0029 |
| HDL-cholesterol | <0.0657 | <0.0034 |
| Creatinine | <0.1142 | <0.0133 |
| Triglycerides | <0.5574 | <0.8989 |
| Urea | <0.4122 | <0.9841 |
| Pupils | <0.0093 | <0.5301 |
| TRYP | <0.0436 | <0.0057 |
| Fatige | <0.0000 | <0.0000 |
| GLN | <0.7398 | <0.8256 |
| VAL | <0.2165 | <0.0085 |
| ILE | <0.1610 | <0.0107 |
| LEU | <0.0428 | <0.0032 |
| TYR | <0.9871 | <0.0804 |
| PHE | <0.0319 | <0.2707 |
| MET | <0.2675 | <0.8352 |
| 3 (val,ile,leu) | <0.0652 | <0.0014 |
| 6 (v-i-l-t-p-m) | <0.0606 | <0.0010 |
| TRYP/BCAA | <0.0077 | <0.0183 |
| TRYP/LCNA | <0.0071 | <0.0175 |
It should also be noted that, under placebo conditions, the cyclists who underwent the laboratory test showed that the trp/LNAA ratios, were numerically consistent with values published by Fernstron and Faller for mice in their study's control group.19,20
We also found changes in pupil diameter 30min after tryptophan ingestion, elicited by identical light stimuli applied prior to physical exercise. This was more marked (increased miosis) in subjects who had not taken tryptophan supplements.
Subjects given tryptophan supplements showed less miosis because of a reduced pupil response after receiving tryptophan supplements. According to the conclusions of 21 (Endorphines et exercise physique. N. Fellmann), administering tryptophan alters the usual pupil response because of the action of endogenous opioids released during physical exercise, with a peak effect 2h post-ingestion, also there were a detectable change after 30min.
Two comments can be made regarding the relationship between tryptophan and cholesterol:
- a)
Firstly, in the cyclist experiment, there was a significant rise in plasma cholesterol levels during exercise. Values rose from x=143.5mg/dl to x=157.2mg/dl in all cyclists given either placebo or tryptophan supplements, The same occurred with the HDL fraction, as also shown in Fig. 2.
- b)
In order to interpret these data, it must be remembered that cholesterol represents a metabolic crossroads, being the starting point for many hormonal substances (corticosteroids, oestrogens, androgens, etc.), vitamins (vitamin D) and substances involved in digestion (bile salts).
Cholesterol is highly sensitive to physical activity, and its metabolism is readily altered according to its duration and intensity. It seems to be the HDL fraction that is responsible for the increase. Ohkuwa22 demonstrated a significant rise in the HDLc fraction during short sessions of intense exercise, with no changes in the LDLc fraction.
Lamon-Fava et al.23 were able to demonstrate a rise in the HDLc and HDL2c fractions at the end of a prolonged competition, as was Kuus.24
Angelopoulos25 showed that 30min of exercise at 65% of VO2 max was enough to cause a significant rise in the HDLc fraction. In our study, plasma cholesterol levels clearly had a big impact on fatigue generation, as shown the second component in importance.
In our experimental trials, plasma triglyceride values were only measured at rest and after 180min of exercise. They were higher with tryptophan supplementation than with placebo, both at rest and post-exertion Fig. 2. Consideration should therefore be given to whether tryptophan supplementation might mean increased bioavailability of triglycerides as body fuel. This issue warrants more in-depth investigation, because there is evidence of changes (significant or otherwise) in the bioavailability of body fuels involved in physical exercise, when subjects are given tryptophan supplements. This leads to perhaps the biggest question raised by this study: the observation that taking tryptophan, at the right dose, alters the usage of body fuels. There is lower consumption of glucose and perhaps glycogen, and enhanced use of triglycerides, although we do not know the detailed mechanisms that explain this effect.
In terms of the side effects of taking tryptophan as an isolated amino acid, no effect on renal function was detected. This is indicated by the behaviour of the creatinine and urea parameters, which showed no significant differences when subjects had taken either tryptophan or placebo (see Fig. 3 and Table 3). Both parameters remained at normal values, both at rest and after exercise, although creatinine showed an upward trend, consistent with a decline in renal function, due to redistribution of the blood supply during prolonged intense exertion (Fig. 3 and Table 3).
In this study, there were no significant differences in haematocrit values between the situations studied (supplementation with tryptophan or placebo), either at rest or during exercise. However, figures were always higher during exercise than at rest, indicating haemoconcentration, despite the hydration regimen maintained during exercise.
Tryptophan supplementation produced a reduction in the levels of branched-chain amino acids (BCAA), overall and each one of them. On the other hand, exercise altered the plasma tryptophan/BCAA ratio, without that entailing greater fatigue in subjects given tryptophan supplements. This is consistent with Varnier26 and the arguments of various authors.27–31
Tryptophan supplementation had a much greater impact on the above-mentioned tryptophan/BCAA ratio, without subjects noticing more fatigue. In fact, quite the opposite was true. This contradicts the belief that the ratio between tryptophan and branched-chain amino acids has something to do with the subject's perceived fatigue levels.
However, it should be taken in consideration that the tryptophan values we used were plasma total tryptophan rather than plasma free tryptophan.
Of the amino acids studied, methionine was the only one for which plasma levels rose in the group given tryptophan supplements. That is interesting, given that it is a methyl donor involved in the formation of creatine, choline and adrenaline (see Fig. 3 and Table 3).
Lastly, to summarise, we accept the model described by Mark Davis.30
Increased brain 5-HT synthesis occurs in response to an increased delivery to the brain of blood-borne tryptophan. Most of the TRP in blood plasma circulates loosely bound to albumin, but it is the unbound or free tryptophan (f-TRP) that is transported across the blood/brain barrier. This transport occurs via a specific mechanism that TRP shares with other large neutral amino acids, most notably the branched-chain amino acids (BCAAs).
Serotonin synthesis will increase when there is an increase in the f-TRP/BCAA ratio. This would occur during prolonged exercise as BCAAs are taken up (oxidised) in skeletal muscles and tryptophan is displaced from its binding sites on albumin by competition from plasma free fatty acids mobilised during exercise.
However, rather than postulating that a parallel rise in L-tryptophan and 5-HT bears a causal relationship to increased perception of fatigue, our research group, in the light of the evidence obtained in our various studies, has formulated another hypothesis. According to our hypothesis, the lower bioavailability of L-tryptophan that occurs during exercise in subjects whose L-tryptophan intake has been restricted is related to earlier onset of fatigue. Conversely, when tryptophan bioavailability is higher, the onset of fatigue is delayed. We also assume that tryptophan has considerable peripheral repercussions related to the bioavailability of body fuels in subjects undertaking prolonged exercise.
Conclusions- 1.
Higher tryptophan levels counteracted the rise in subjective perception of fatigue during the performance of physical exercise (the Borg SPF test). In principal component analysis, the impact of tryptophan was estimated to be 4% in our model (Table 4). Tryptophan supplementation does not affect renal function. Tryptophan supplementation alters pupil size. At the doses administered, tryptophan supplementation proved effective for the first 2h following the last intake of the tryptophan supplement.
- 2.
At the appropriate dose, tryptophan supplementation seems to alter the bioavailability of body fuels, favouring the use of triglycerides rather than glucose during exercise.
- 3.
The results obtained suggest that tryptophan is somehow involved in mechanisms that raise the malaise/pain threshold, so the onset of sensations of fatigue, malaise and pain are delayed during prolonged exercise.
- 4.
A classical explanation attributes alterations in fatigue perception to changes in neurotransmitter synthesis and beta-endorphin production. A second explanation can be added at the muscle level. The first explains how tryptophan supplementation permits changes in the function of systems for the uptake and production of signals associated with pain, defined as anything from simple malaise or discomfort to really intolerable sensations. Neurotransmitters influence the synthesis and/or release of endorphins, which are endogenous opioids that affect pain tolerance levels without otherwise distorting normal sensory perception. According to the second explanation or model, by some unknown mechanism tryptophan hinders the mobilisation of muscle glycogen stores, thus forcing the body to use fats as fuel by the aerobic pathway. The non-depletion of muscle glycogen stores prevents the sensations of malaise caused by this depletion.
- 5.
When the pupils of the cyclists’ eyes were observed, we found evidence of different responses between the two test trials (with tryptophan or placebo supplementation), beyond the blood/brain barrier, in the brain; we attribute this to the levels of tryptophan intake within the last 24h.
Department of Physiological Sciences, Medical School, University of Barcelona, Barcelona, Spain.
Conflict of interestThe authors declare that they do not have any conflict of interests.