To evaluate the accuracy of shear wave elastography (SWE) ultrasound in the assessment and quantification of fatty infiltration (FI) of the supraspinatus (SSP) muscle with the Goutallier classification on CT as the reference standard.
Materials and MethodsProspective study of 90 patients from December 2020 to August 2022 who had undergone shoulder US including SWE for the assessment of rotator cuff disease and had a previous CT with the shoulder included. Shear modulus (in meters per second) was estimated using ultrasound SWE in three regions of the SSP (myotendinous junction, anterior and posterior muscle bundle). Data collected included age, sex, and fatty muscle infiltration on CT (Goutallier stages 0–IV). Pearson correlation between CT Goutallier stage of the FI of the supraspinatus and SWE value in each region of the supraspinatus muscle was determined. Bland-Altman indexes were used to assess agreement between readers.
ResultsThere were statistically significant differences in the SWE and the different Goutallier groups in the SWE of anterior bundle muscle, posterior bundle muscle, and myotendinous junction (p = < 0.001, 0.008, 0.031, respectively). The differences were significant for SWE of the anterior bundle muscle among the different subgroups of Goutallier stage (I-IV, p = < 0.001).
ConclusionSWE shows an excellent correlation with the Goutallier stage on CT in detecting, qualifying, and quantifying fatty infiltration of the supraspinatus. SWE may be useful in quantifying supraspinatus muscle quality in athletes competing in racquet sports and in other sports with overhead motions such as swimming, volleyball and javelin.
Chronic rotator cuff tears are associated with irreversible histological modifications of the musculotendinous unit, such as muscle fatty infiltration, increased interstitial connective tissue, and tendon retraction.1 Fatty infiltration (FI) is a significant predictive factor for surgical outcome.2–5 Preoperative evaluation of fatty infiltration is critical for planning operative treatment and, above all, for predicting successful rotator cuff repair. Advanced FI of the supraspinatus is associated with high rates of failure and worse clinical outcomes after rotator cuff repair.6,7
Different imaging techniques have been used to detect the FI of the supraspinatus. Even though the original Goutallier grading system (0–4) was based on axial CT images, MRI has become the accepted modality for evaluating and classifying fatty infiltration of the SSP.8 Previous publications have demonstrated the utility of ultrasound in assessing atrophy and fatty infiltration of the supraspinatus muscle, showing a good correlation between MRI and US for assessing rotator cuff abnormalities.9–11 In addition, ultrasound has potential advantages over MRI because of its cost-effectiveness and broader availability.
Ultrasound applications enable the acquisition of quantitative results for the analysis of fatty infiltration. Recent studies highlight the growing significance of quantitative muscle ultrasound (QMUS) and shear wave elastography, particularly allowing for detailed characterization of muscle quality and pathology.12,13 QMUS techniques, such as echogenicity analysis and calibrated backscatter analysis, focus on providing quantitative data related to tissue composition, allowing for discrimination between normal muscle and muscle pathology. On the other hand, shear wave elastography (SWE) provides a quantitative assessment of musculoskeletal tissue integrity, focusing on tissue stiffness and elasticity.14,15 This method is based on so-called shear waves generated using a focused acoustic radiation force from a linear US array that interacts with the tissues and generates horizontally directed shear waves. The velocity of these shear waves can be quantified by using ultrafast algorithms to evaluate tissue properties and elasticity.14 SWE has been shown as a helpful technique in rotator cuff abnormalities such as tendon softening with increasing rotator cuff tendinopathy16–18 or rotator cuff tear19–21 and in patients with adhesive capsulitis.22 In addition, SWE has been applied to assess muscle diseases and non-pathologic disorders, including age-related changes in muscle stiffness.23
The primary objective of this study to determine how estimated supraspinatus shear modulus (SWV) in three SSP muscle zones (myotendinous junction, anterior muscle bundle, and posterior muscle bundle,24,25 is correlated with the CT Goutallier classification of FI of the rotator cuff, grayscale indices and muscle quality. A secondary objective was to use SWE to quantify the degree of fatty degeneration of the supraspinatus muscle.
Materials and methodsStudy designThis was a prospective study performed in the Department of Radiodiagnosis and Imaging.
The institutional ethics committee approved the study (approval no. 2021.148). Informed consent was obtained from all patients. Clinical data collected included patient age, gender, and arm dominance.
Consecutive patients who referred for shoulder US from the department of orthopedics with suspected rotator cuff injury between December 2020 and August 2022 were recruited for this prospective study with the goal of recruiting a total of 95 patients.
Patient recruitment and ultrasound examinations were performed at the same hospital. The inclusion criteria were age ≥ 18 years, referral for shoulder ultrasonography for shoulder pain, CT exam performed withing the preceding 12 months for an annual medical check-up program which included a dual-source 128-MDCT of the chest (Somatom Drive, Siemens Healthineers) which included the shoulder. Exclusion criteria for the study included known supraspinatus muscle disease process not related to rotator cuff disease; prior joint replacement surgery or rotator cuff surgery; the presence of cervical radiculopathy or other central or peripheral neurologic deficits of the upper limb.
Imaging evaluationUS evaluation of the supraspinatus muscleAll shoulder US examinations were performed with a Logiq S8 R4 (General Electric, United States) using a single linear array transducer (9 to 15 MHz).
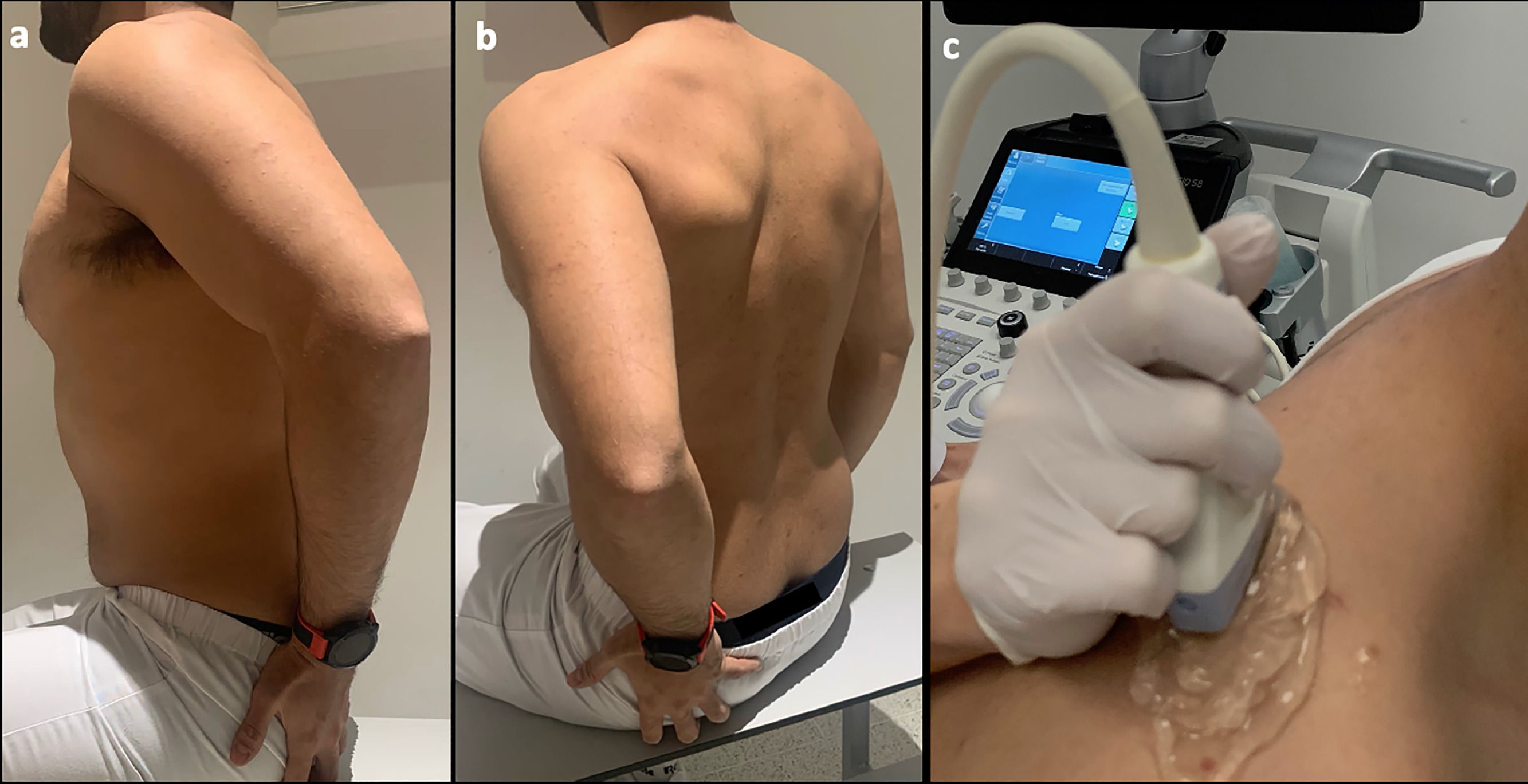
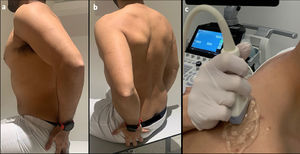
All ultrasounds were performed by a single radiologist with 10 years of experience as a dedicated musculoskeletal radiologist who was blinded to the CT findings. All patients were scanned with the arm in the Crass position,26Fig. 1 with the palm of the hand placed on the superior aspect of the gluteal region and with the elbow flexed (resulting in mild internal rotation of the humerus). This position has become the standard technique for evaluating the supraspinatus tendon because of its excellent visualization of supraspinatus abnormalities.
Photograph showing example scanning position and plane for shear wave acquisition. (a,b) The sitting position is shown, with the arm in the Crass position. The arm posteriorly placing the palmar side of the hand on the superior aspect of the iliac wig with the elbow flexed (internal rotation). (c) The probe position is shown longitudinal orientation relative to the supraspinatus muscle. During data acquisition, an amount of gel (5 mm) and minimal contact pressure were applied to minimize the longitudinal preload.
The SSP muscle was examined at its widest point. Grayscale images (b-mode) were obtained in both long and short axes holding the transducer parallel and perpendicular to the long axis of the muscle belly, respectively. The maximum diameter of the supraspinatus muscle was sought in the glenoid fossa. After selecting this area, a shear wave ultrasound measurement (SWV) of the supraspinatus muscle was performed in longitudinal plane parallel to the supraspinatus muscle fiber orientation.27 Imaging in this plane correlates strongly with muscle biomechanical properties and is less sensitive to the inherent viscoelastic nature of the soft tissue.28–30 The SWV [in meters per second (m/s)] was assessed in the SSP muscle using dedicated SWE software from GE Healthcare (Milwaukee, WI, USA) on a GE Logiq S8 scanner. SWE was performed on each participant's involved shoulder in three regions (myotendinous junction, anterior and posterior muscular bundle.24 Initial color maps were selected to confirm that the SWV was within the range of detectable velocities, previously determined to be 0.0–12 m/sec as indicated by the manufacturer.
SSP muscle SWE properties were evaluated by placing a region of interest (ROI) in these 3 muscular regions: myotendinous junction, anterior muscle bundle, and posterior muscular bundle. In the longitudinal plane, the SSP shows an intramuscular tendon which could be detected as a homogeneously hyperechoic structure in the bipennate anterior fiber bundle. On the other hand, the posterior bundle is a unipennate muscle bundle with no intramuscular tendon and has a more parallel fiber arrangement.
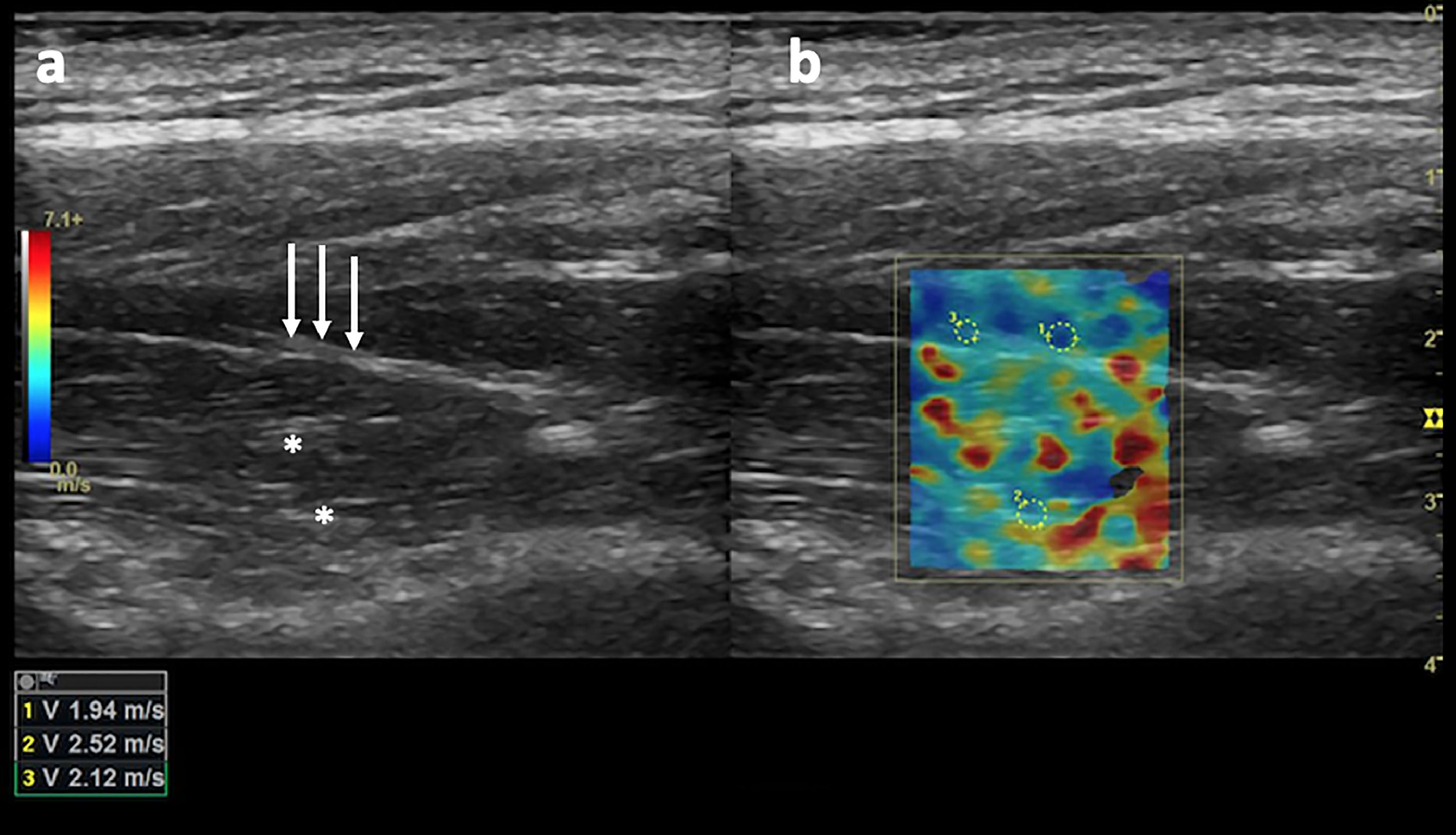
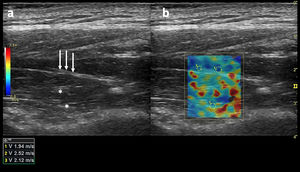
Subsequently, SWV was measured in meters per second in each region of interest (with a minimum of 1 cm between ROIs) by placing the ROI in a shadow-free region and confirming that the ROI had color-fill with the default gain settings (55, Fig. 2). Images that did not contain a portion of the ROI meeting these criteria were deemed unacceptable and thus rejected.
(a) Long-axis grayscale US image of the supraspinatus muscle belly. Central tendon (long arrows) and pennate pattern are visible. There is mildly increased echogenicity relative to normal background muscle (asterisks). (b) SWE image (color elastogram) of the same region shows predominantly intermediate shear-wave velocity on anterior (1), posterior (2) and myotendinous junction (3,1.94 m/sec, 2.52 m/sec, 2.12 m/sec, respectively). Red = hard consistency (7.1 m/sec), blue = soft consistency (0.0 m/sec), and green and yellow = intermediate consistency. SWE data were collected using a Logiq S8 R4 (General Electric, United States) with an L9–15-MHz linear transducer.
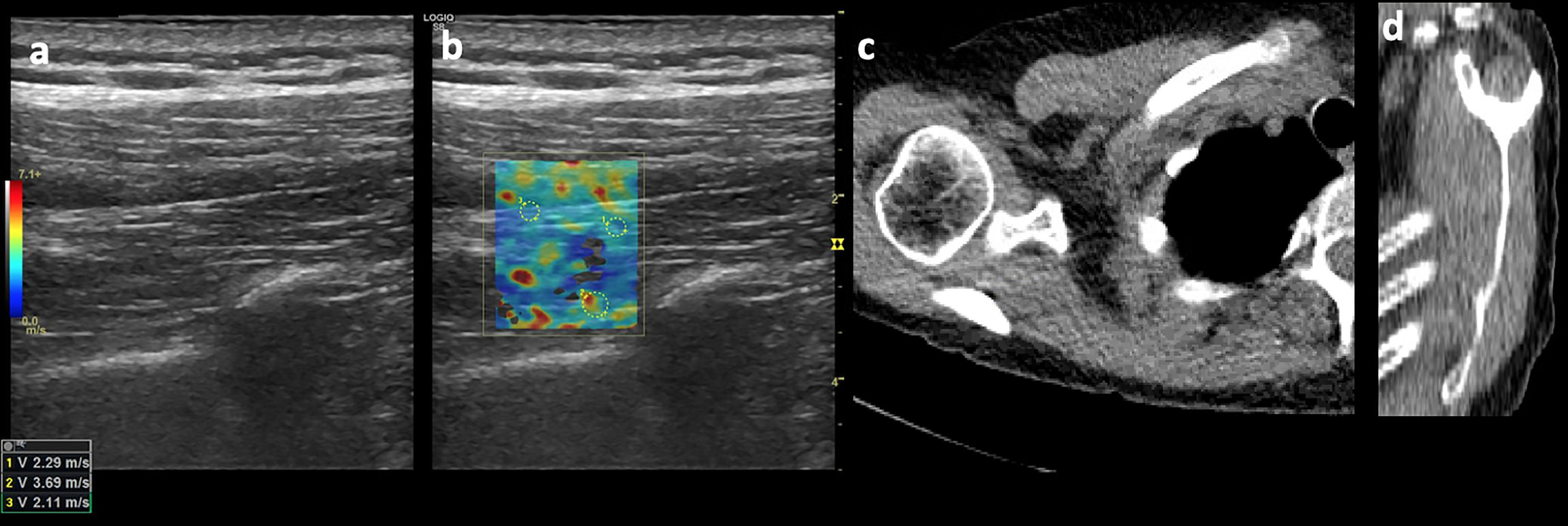
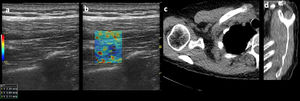
A commercially available PACS (Vue PACS, Carestream) with standard soft-tissue window settings (width, 350 HU; level, 50 HU) was used to view the chest CTs images. DICOM image modules were created containing a single oblique sagittal CT image corresponding to the Y-shaped view for each shoulder (Fig. 3). The grade of fatty atrophy of the supraspinatus muscle belly was assessed according to the Goutallier classification and was graded as follows: stage 0, no fatty streaks; stage I, some fatty streaks; stage II, fatty infiltration but less fat than muscle; stage III, equal amounts of fat a muscle; and stage IV, more fat than muscle2 (Fig. 3).
Images of a 45-year-old male patient. (a) Long-axis grayscale US image of supraspinatus muscle belly with increased echogenicity relative to normal background muscle. (b) SWE image (color elastogram) of the same region shows predominatly low intermediate shear-wave velocity on anterior (1), posterior (2) and myotendinous juction (3,2.29 m/sec, 3.69 m/sec, 2.11 m/sec, respectively). Red = hard consistency (7.1 m/sec), blue = soft consistency (0.0 m/sec), and green and yellow = intermediate consistency. SWE data were collected using an Logiq S8 R4 (General Electric, United States) with a L9–15-MHz linear transducer. (c) Axial CT shoulder image used to quantify the amount of fatty degeneration of the supraspinatus shows degeneration of some fatty streaks within supraspinatus (Goutallier grade II). (d) Sagittal oblique CT image shows supraspinatus with moderate atrophy and fatty degeneration (Goutallier grade II).
SSP fatty infiltration grading on CT images and SWE imaging were assessed independently by two readers (Reader 1, 14 years of experience as a musculoskeletal radiologist; Reader 2, 10 years of experience as a musculoskeletal radiologist). The CT readers were blinded to the SWE findings.
Data analysisThe statistical analyses were computed with IBM SPSS software (version 22.0; IBM, Armonk, NY). Statistical significance was defined as p < 0.05. For descriptive analysis, the quantitative parameters were evaluated by calculating the mean value, SD, and range, and the categoric parameters were assessed by determining the absolute and relative frequencies.
Linear-weighted kappa coefficient was used for determining intraobserver agreement. Lineal weighted kappa was computed using the StatXact statistical program (version 11.0.0 for windows, Cytel Studio 11, Cytel Inc., Cambridge, MA, United States).
Interobserver agreement on SWE SSP in three regions (myotendinous Junction, anterior and posterior muscular bundle) was evaluated for each reader pair using intraclass correlation and Bland-Altman indexes. Bland Altman indexes were performed using GraphPad Prism (version 9.0.0 for Windows, GraphPad Software, San Diego, California USA, www.graphad.com). Correlation between the stiffness of the supraspinatus muscle image measurements (myotendinous junction, anterior and posterior muscular bundle) on SWE and age was determined using Pearson correlation coefficients. The relation between the stiffness of the supraspinatus muscle image measurements (myotendinous Junction, anterior and posterior muscular bundle) on SWE and sex was determined using a t-test. Comparison between the estimated SWE of the supraspinatus muscle (myotendinous junction, anterior and posterior muscular bundle) and Goutallier grades were analyzed using univariate analysis of variance (ANOVA), the Scheffé test as post hoc analysis, and the independent- samples t-test. Statistical significance was defined as P < 0.05. Violin plots were used for illustration. Eta-squared was used for ranking substantial contributions to the overall error. The eta-squared is the proportion of the total variance attributed to an explanatory variable.31
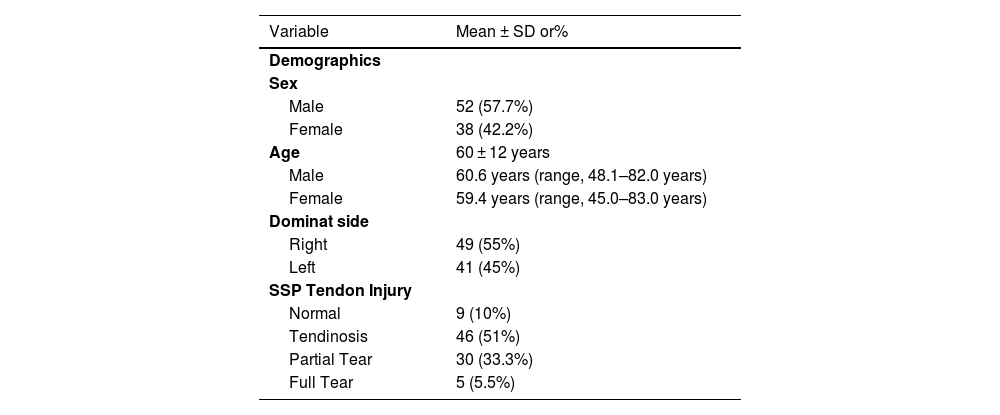
ResultsPatientsOf a total of 105 potential patients according to selection criteria, 10 refused to participate and 95 patients were initial enrolled. However, 5 patients were subsequently excluded due to evidence of prior ipsilateral shoulder surgery. Therefore, the final study sample consisted of 90 patients: 38 females and 52 males with a mean age of 60 ± 12 years (range 37–83). Demographic data are shown in Table 1.
Demographic data for all patients (n = 90).
SSP: supraspinatus.
All ultrasound examinations were technically adequate with proper visualization of the three components of the SSP and color maps of the SWV were within the range of detectable velocities (as indicated by the manufacturer) in all patients.
ReliabilityIntraobserver reliability was excellent for Goutallier classification based on CT of the SSP muscle (ICC=0.992; 95% CI: 0.987, 0.997; p = 0.001).
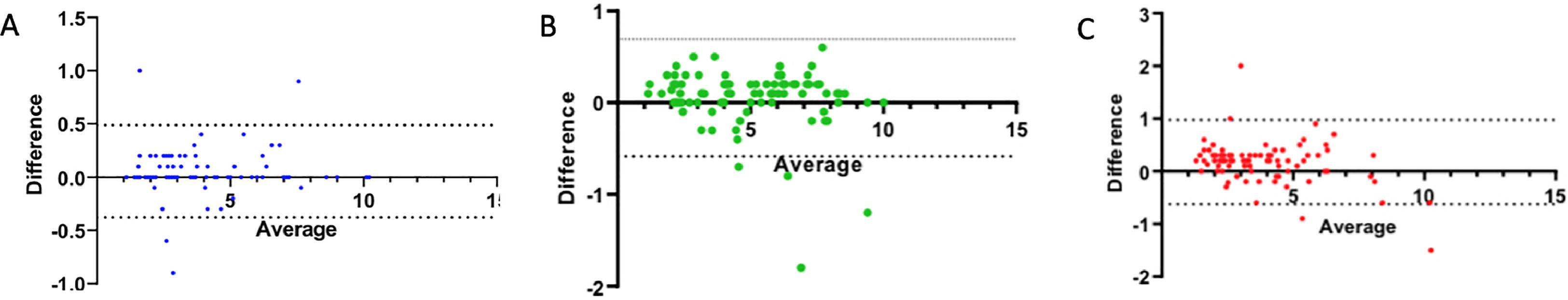
Interobserver agreement between the two readers assessed by the Bland-Altman analysis demonstrated a positive bias, with a mean bias of 0.17 ± 0.40 (95% CI: −0.62 to 0.97) for measurements of SWE SSP at the myotendinous joint. A positive bias of 0.05 ± 0.22 (95% CI: −0.37 to 0.48) and 0.05 ± 0.32 (95% CI: −0.58 to 0.69) was also evident for measurements SWE SSP in the anterior bundle and posterior bundle, respectively (Fig. 4).
Bland-Altman analysis reveals minimal proportional bias between readers and SWE on each region of SSP relative to fatty infiltration. Bland-Altman (mean-difference) plots show the relationship between average readers (R) and the difference between R for SWE SSP for anterior bundle (A), posterior bundle (B) and myotendinous junction (C), respectively . The solid black line shows the mean bias for each comparison. Dashed lines represent the limits of agreement (95% confidence interval) around the mean bias (0.22,0.32,0,40 for A,B and C standard deviation [SD]). SSP = Supraspinatus, SWE = Shear-Wave Elastography.
There was no correlation between age and SWE of the SSP muscle in the anterior bundle (r = −0.024, p = 0.823), age and SWE of the SSP muscle in the posterior bundle (r = 0.070, p = 0.509), or age and SWE of the SSP at the myotendinous junction (r = −0.029, p = 0.786). There was no statistically significant sex dependence for SWV of the SSP muscle at the anterior bundle, posterior bundle and myotendinous junction, respectively (male, p = 0.56, p = 0.91, p = 0.13; female, p = 0.56, p = 0.92, p = 0.14).
Distribution of the CT Goutallier classification was as follows: Goutallier stage 0 (n = 0), Goutallier stage I in 23,3% of patients (n = 21), Goutallier stage II in 48,8% (n = 44), Goutallier stage III in 22.2% (n = 20) and Goutallier stage IV in 5.5% (n = 5). The median Goutallier stage was 2 (range, 1–4).
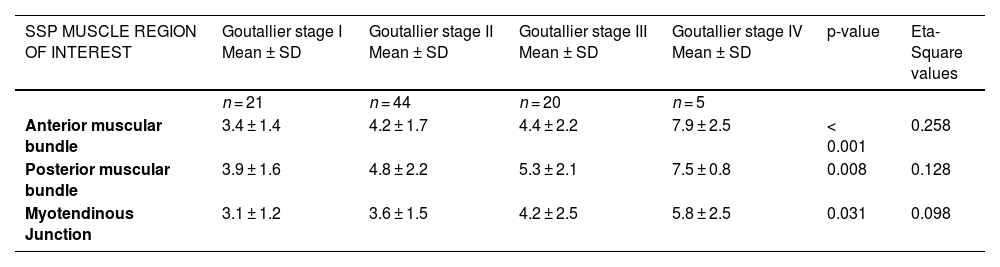
Univariate ANOVA showed a statistically significant difference in the SWE and the different Goutallier groups (P values between 0.051 and >0.99) in the SWE of the anterior bundle muscle (p < 0.001), posterior bundle muscle (p = 0.008), and myotendinous junction (p = 0.031) (Table 2). The eta squared statistics have a value of 0.258 comparing SWE of the anterior bundle muscle with the different Goutallier groups, 0.128 in the posterior bundle muscle and 0.098 at the myotendinous junction.
SWE of the SSP Muscle in Relation to Fatty Infiltration.
Note.—Except for P values (obtained with ANOVA) and ETA-Square values, numbers are mean SWVs in meters per second ± standard deviations.
SSP: Supraspinatus.
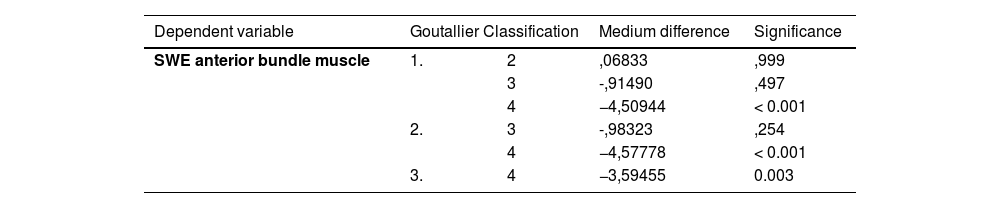
There was a good correlation between SWE in the anterior bundle of the SSP muscle and Goutallier classification (Eta = 0.258; p < 0.001). The post hoc Scheffé test showed statistically significant differences for SWE among the different subgroups of Goutallier stage I-IV and the anterior bundle muscle. (p = < 0.001; Scheffé corrected significance level p = 0.005) (Table 3).
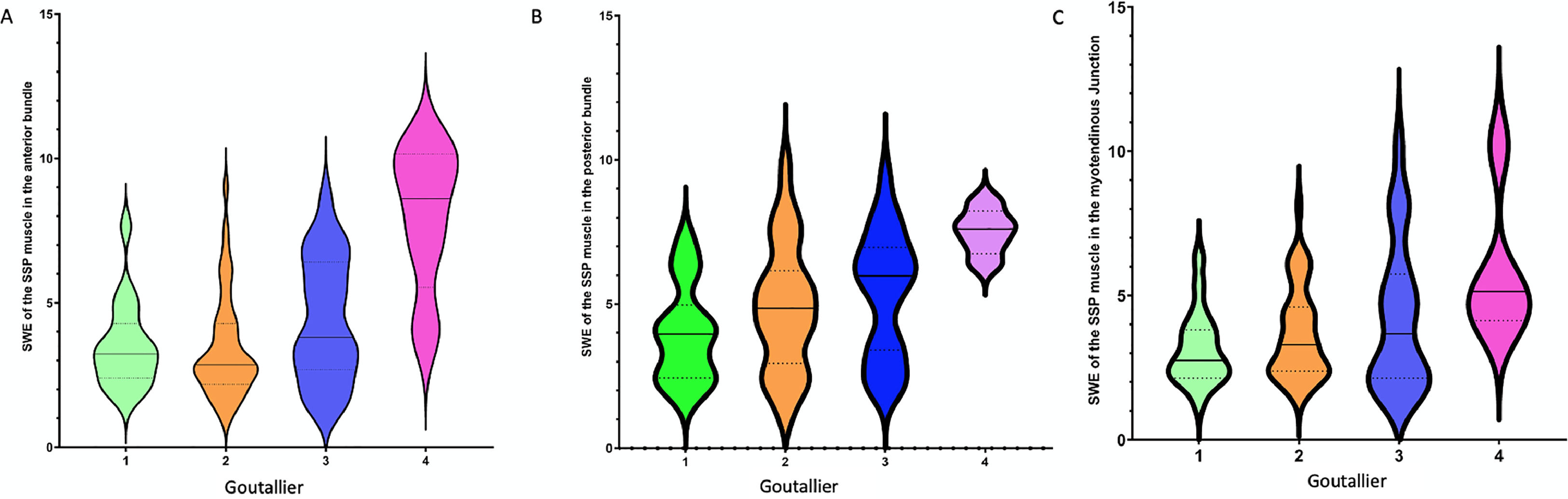
Violin plots were used to compare muscle fatty infiltration according to CT Goutallier Classification and SWE in the three regions of SSP (Fig. 5).
Violin plots represent SWE of the SSP muscle in the anterior bundle (Y-axis) and Goutallier staging in patients (X-axis). The violin plot outlines illustrate density of data at each point along the reported range, that is, the width of the shaded area represents the proportion of the data located there whereas the skinnier sections represent a lower probability. (A) The horizontal line within each plot indicates median SWE of the SSP SSP muscle in the anterior bundle. (B) Violin plots represent SWE of the SSP muscle in the posterior bundle (Y-axis) and Goutallier staging in patients (X-axis). (C) Violin plots represent SWE of the SSP muscle in the myotendinous junction (Y-axis) and Goutallier staging in patients (X-axis).
Since fatty infiltration (FI) is a predictive factor for the outcome of surgical rotator cuff repair the present study aimed to quantitatively assess fatty infiltration of the supraspinatus by shear-wave ultrasound elastography in cases of suspected rotator cuff injury, correlating it with the Goutallier imaging classification and its potential applicability in the assessment of athletes. The principal findings of the study is the good correlation between SWE in the anterior bundle of the supraspinatus muscle and Goutallier classification. These findings provide valuable insights into the relationship between Goutallier classification and SWE in patients with suspected rotator cuff tears, which can potentially have implications for the assessment of athletes and other individuals with rotator cuff injuries. In certain sports, such as racquet sports and other sports that require an overhead throwing motion, such as swimming, volleyball and javelin, this technique may be useful in assessing changes in supraspinatus muscle composition/stiffness resulting from traction or compression injuries to the suprascapular nerve.
A recent study by Jeong et al. confirms the usefulness of preoperative SWE of the supraspinatus muscle in predicting the success of rotator cuff repair as compared to MRI.32
It has been postulated that fatty infiltration increases with the severity of the SSP tear. As the rotator cuff tear increases in size, the associated myotendinous retraction results in a change in the pennation angle of the supraspinatus muscle fibers with respect to the tendon. Previous studies have shown that the greater the change in the pennation angle, the greater the amount of fatty infiltration and muscle atrophy.33,34
Gimbel et al. found a time-dependent change in the SSP tendon stiffness in rats after detachment of the corresponding tendon. The stiffness decreased slightly during the first two weeks and increased from week 3 to 16.35 SWE may be able to detect these mechanical changes in the muscle due to fibrosis not readily assessed visually on morphologic imaging.33 Gilbert et al. demonstrated that shear wave velocity increased as fat content in the muscle (as demonstrated by MR spectroscopy) increased.36
Our study demonstrates increased SWE in the SSP muscle with increasing fat at CT imaging (Goutallier stage I-IV). In fact, our results show a striking increase SWE in Goutallier stage IV. This result is consistent with the findings by Rosskopf et al.17 who reported that the SWE in the SSP was 2.4 m/sec ± 0.3 for Goutallier stage IV infiltration.
In our series, there was a significant correlation between the SWE of the supraspinatus muscle and the Goutallier stage on CT to detect fatty infiltration of the supraspinatus muscle in the anterior bundle muscle (P = 0.000), the posterior bundle muscle (P = 0.008) and the myotendinous junction (P = 0.031). These finding are similar to the previously reported rates by Itoigawa et al.,25 which also have a significant correlation between the stiffness of the supraspinatus muscle and the Goutallier stage on MRI (R 1⁄4 0.48, P < 0.01).
Nevertheless, Lawrence et al. reported that shear modulus was not significantly different between Goutallier grades under any testing condition (P > 0.30,20). However, that study only included patients scheduled for arthroscopic rotator cuff repair with minor or medium-sized tears. Therefore, the degree of muscle fatty degeneration in patients with massive or large tears was not assessed, potentially affecting their ability to find potentially significant relationships.
Similarly, a study by Seo et Al., investigating the correlation between qualitative sonoelastography and Goutallier classification of the SSP muscle on MR images concluded that muscle tissue becomes softer with increasing Goutallier stage.37 Although that study's results differ from ours, there are severe differences between the studies that may explain the apparent discrepancy, including differences in probe orientation and our use of quantitative sonoelastography (SWE) versus CT as the modality for grading muscle fatty infiltration.
Furthermore, FI of the SSP is associated with multiple factors such as the chronicity of the tear, patient age, tear size, and location of the injury.38,39 Our results demonstrate that the FI developing in the anterior aspect of the SSP bundle had a good correlation between SWE in the anterior bundle of the SSP muscle and its Goutallier classification (P = < 0.001; Scheffé corrected significance level = 0.005). This is consistent with the findings of Kim et al. The probability of fatty degeneration developing in the supraspinatus increases when a tear involves the more anterior aspect of the supraspinatus tendon (r = 0.70).40 It has been theorized that FI of the SSP most commonly affects the anterior part of the SSP tendon. There are many possible explanations for this finding. Degenerative rotator cuff tears typically begin at the anterior portion of the supraspinatus insertion near the biceps.23 Furthermore, the anterior SSP tendon is thicker and more tubular, whereas the posterior tendon is short and thin. Disruption of this anterior tendinous portion can lead to significant muscle damage, including biological and mechanical changes.41
LimitationsThe current study has several limitations. First, the precise degree of FI is unknown without a histologic correlation as a standard of reference for the FI of the SSP muscle. Consequently, we applied the Goutallier classification system, which has been widely used in clinical studies on shoulder pathology as a standard of reference to assess the fatty infiltration.2,42 Second, the subgroup of patients with Goutallier stage IV fatty degeneration was small (n = 5). However, the analyzed group represents patients who may benefit less from shoulder surgery.43 Third, the study specifically focused on patients with suspected rotator cuff tears and, perhaps as a result of that indication, there were no patients with Goutallier stage 0. Fourth, fatty infiltration of the supraspinatus could be attributed to various factors, including denervation, diet, genetics, and other co-morbidities, which were not recorded during the study.44 This could limit the generalizability of the results. However, despite gathering information about other factors like age, sex or dominance, the analysis did not reveal any statistically significant differences of the SWE in the three regions of supraspinatus. Fifth, patients’ physical activity 30 min prior to the shear wave ultrasound were not taken into consideration during the analysis. Previous articles have shown physical activity can indeed have an impact on shear wave values in supraspinatus muscle.14,45 Sixth, regarding the lack of cut-off values for SWE measures of fatty infiltration in rotator cuff, the results of this study would be in line with previously published studies17,32 which suggest that in healthy individuals, SWE values are greater than those in patients suffering from rotator cuff disease.
ConclusionShear wave elastography ultrasound is an excellent reproducible technique for assessing and quantifying fatty infiltration of the supraspinatus. SWE shows a good correlation with the Goutallier stage on CT in detecting and quantifying fatty infiltration in the SSP (anterior bundle muscle, posterior bundle muscle, and myotendinous junction). Specifically, the highest correlation between SWE and fatty infiltration was seen in the anterior bundle of the SSP. SWE may useful in quantifying supraspinatus muscle quality (atrophy and stiffness) in athletes not only in the setting of rotator cuff tears but potentially in the setting of traction/compression injuries of the suprascapular nerve such as those that may be seen in racquet sports and in other sports with overhead motions such as swimming, volleyball and javelin.




![Bland-Altman analysis reveals minimal proportional bias between readers and SWE on each region of SSP relative to fatty infiltration. Bland-Altman (mean-difference) plots show the relationship between average readers (R) and the difference between R for SWE SSP for anterior bundle (A), posterior bundle (B) and myotendinous junction (C), respectively . The solid black line shows the mean bias for each comparison. Dashed lines represent the limits of agreement (95% confidence interval) around the mean bias (0.22,0.32,0,40 for A,B and C standard deviation [SD]). SSP = Supraspinatus, SWE = Shear-Wave Elastography. Bland-Altman analysis reveals minimal proportional bias between readers and SWE on each region of SSP relative to fatty infiltration. Bland-Altman (mean-difference) plots show the relationship between average readers (R) and the difference between R for SWE SSP for anterior bundle (A), posterior bundle (B) and myotendinous junction (C), respectively . The solid black line shows the mean bias for each comparison. Dashed lines represent the limits of agreement (95% confidence interval) around the mean bias (0.22,0.32,0,40 for A,B and C standard deviation [SD]). SSP = Supraspinatus, SWE = Shear-Wave Elastography.](https://static.elsevier.es/multimedia/26665069/0000005900000222/v1_202405070524/S2666506924000026/v1_202405070524/en/main.assets/thumbnail/gr4.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)



