Exercise induces modifications in thermal homeostasis. The type of exercise may have a specific impact on skin temperature (Tsk).
ObjectiveTo analyze and compare the behavior of Tsk in a resistance training between men and women and monitor the thermal recovery response.
Material and MethodsSixteen male and female adults (24.56±3.22 years old) underwent a resistance circuit training session. They performed 3 sets of 12 repetitions with 70–80% 1 RM for lat pulldown, leg press, and biceps arm curl exercises. Thermograms were taken in anterior and posterior body view at rest, 20min, and 24h after training. Tsk was measured in the body regions of interest corresponding to the brachial biceps, quadriceps, and upper back. ANOVA with Tukey's post hoc test was used to analyze Tsk changes among moments, and independent samples t-test was used to compare Tsk between males and females.
ResultsAt rest, women showed Tsk significantly lower than men. There was no significant Tsk change 20min after exercise. However, Tsk increased 24h after exercise in the upper back for men compared with baseline and in quadriceps for women compared with baseline and 20min after exercise (p<0.05).
ConclusionThe Tsk of women at rest is lower than that of men. A resistance circuit training session does not significantly change Tsk 20min after exercise, but it increases Tsk 24h after training in the upper back for men and quadriceps for women.
Physical exercise changes the body's thermal response.1,2 It is interesting to understand how the different types of exercise impact thermal adjustments to find alternatives that facilitate the control of body temperature. Infrared thermography (IRT) records the heat radiated by a body, allowing us to understand the thermoregulatory adjustments during exercise.3 IRT has been used to study thermoregulatory responses mainly related to aerobic and cyclical exercises.4–8 However, in the case of strength exercises and especially with women, the density of studies on the subject is unusual.
In a search carried out in April 2020 in the PubMed database using the keywords “thermography and exercise”, with the filter “humans”, it was possible to find 134 articles, with only five studies on IRT and strength training.9–13 Among these studies, only one was conducted with women.12 Another interesting aspect is that in the methodology used by these five articles,9–13 none of them was carried out with full training sessions. The authors used protocols with only one exercise to verify the skin temperature (Tsk) response during, just after, or in the recovery period, making it necessary to evaluate the Tsk response resulting from a complete circuit-based strength exercise session. Moreover, it was possible to observe that most of the studies used men as volunteers, probably for methodological reasons.
In this work, it has been proposed to analyze the thermal response 24h after exercise as a possible tool to control the training load.14 IRT could help to understand the local thermal behavior induced by the effect of exercise on cellular regenerative responses through inflammatory and local anabolic responses, such as the recovery of muscle glycogen and the phosphate system.15 Some studies have found an interesting relationship using this approach with soccer players,14,16 who showed that the active body regions were warmer 24h after exercise compared to the resting conditions. This would mean that this body region would still be in the recovery phase, requiring to return at rest Tsk values for performing a new training load.
Since strength training is one of the most important means to improve physical fitness, it is important to know how this process occurs because it differs from the type of aerobic exercise due to its bioenergetic specificities. This study aims to increase the basic scientific knowledge, thus expanding the level of scientific evidence on thermoregulatory adaptations of strength training, especially with a subgroup of women. Therefore, the objective of this study was to analyze and compare the behavior of Tsk between men and women after a session of circuit-based resistance exercise, as well as to analyze the effect of this type of exercise after a 24-h recovery period.
Materials and methodsParticipantsSixteen physically active adults participated in this study (8 males and 8 females) (18–30 years old). All were enrolled in strength training in the last 2 months and were classified as apparently healthy subjects, with negative responses to all PAR-Q questions,17 and classified as having a below-average coronary risk.18 Throughout the data collection protocol, the Brazilian ethical standards for research with human beings were followed according to Law 466/12, which is in line with procedures and methods used for the ethical standards of experiments with humans proposed by the Declaration of Helsinki.19
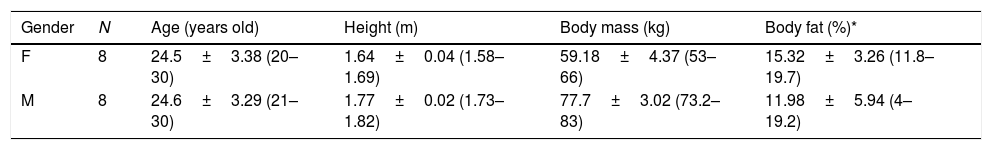
The following exclusion criteria were considered: smoking subjects, musculoskeletal injuries in the last two months, skin burns (in the analyzed body regions), pain symptoms in the evaluated areas, skin allergies, sleep disorders, fever in the last seven days, medical or physical therapy with creams, ointments or lotions, as well as the use of antipyretics and/or diuretics, any nutritional supplement that could change the water body homeostasis or body temperature in the last two weeks, or eating disorders (bulimia and anorexia).20Table 1 shows the anthropometric characteristics of the participants.
Anthropometric characteristics of male and female participants. M=male; F=female.
| Gender | N | Age (years old) | Height (m) | Body mass (kg) | Body fat (%)* |
|---|---|---|---|---|---|
| F | 8 | 24.5±3.38 (20–30) | 1.64±0.04 (1.58–1.69) | 59.18±4.37 (53–66) | 15.32±3.26 (11.8–19.7) |
| M | 8 | 24.6±3.29 (21–30) | 1.77±0.02 (1.73–1.82) | 77.7±3.02 (73.2–83) | 11.98±5.94 (4–19.2) |
Proposed by Jackson and Pollock.21 three skinfolds (chest, abdomen, and thigh for men, and triceps, suprailium, and thigh for women).
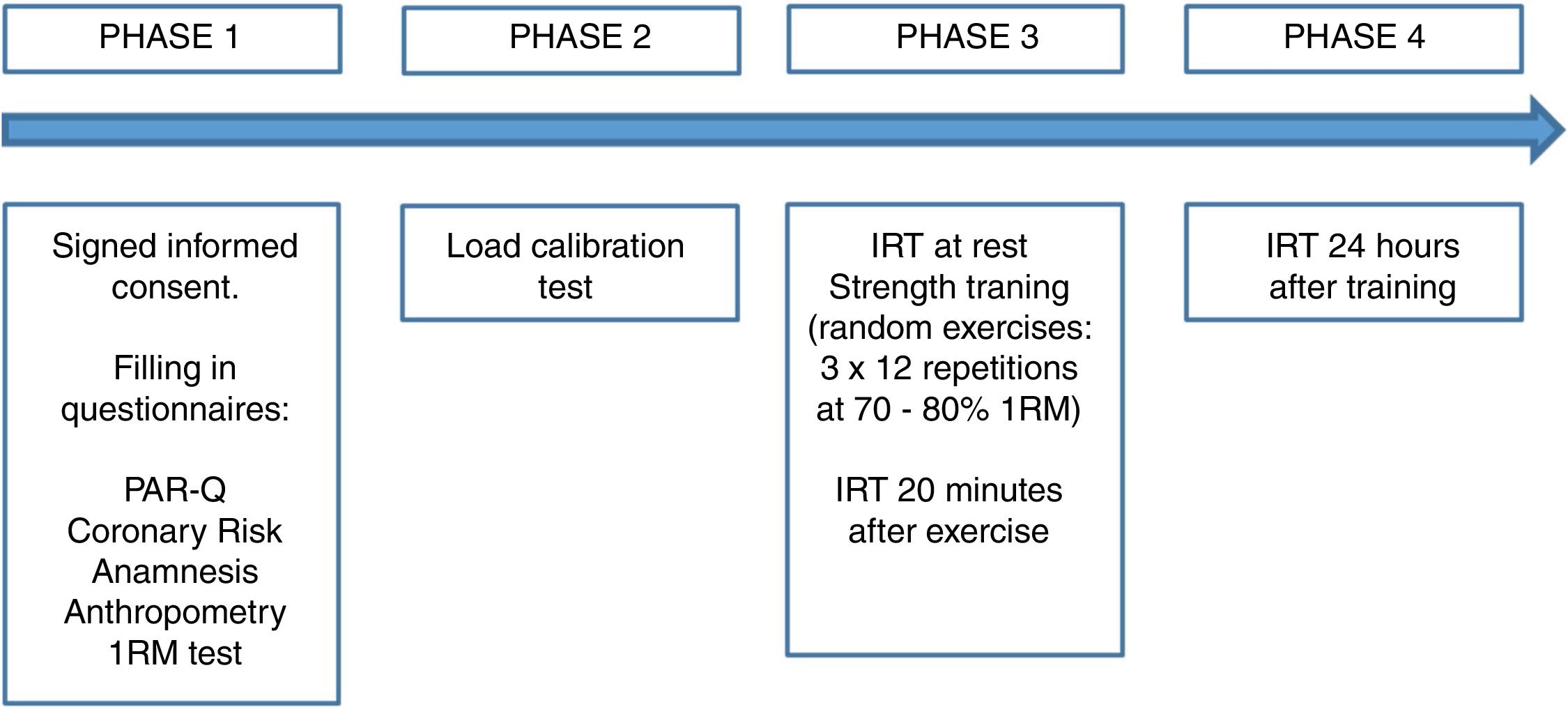
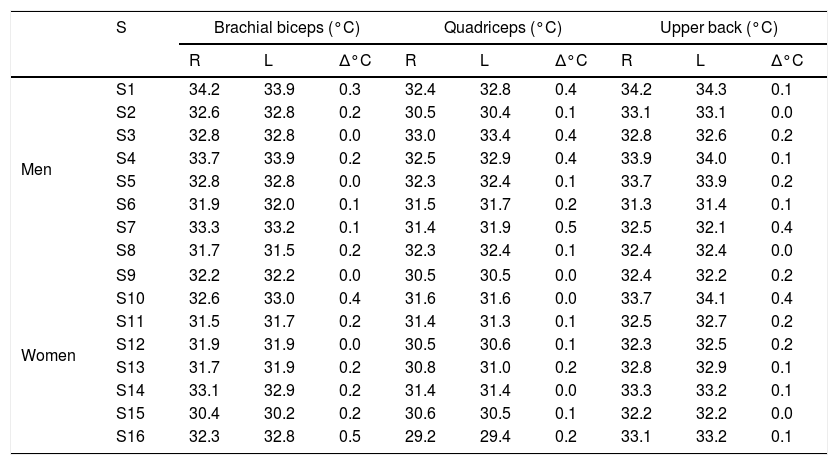
Fig. 1 shows the four phases in which the study was developed.
In PHASE 1, an anthropometric assessment was performed to characterize the participants. Moreover, the 1RM test was carried out following the recommendations proposed by Marins and Giannichi,22 in three exercises: dumbbell biceps arm curl, lat pulldown, and leg press. First, a 10-min organic warm-up was performed on a cycle ergometer. Subsequently, participants were familiarized with the strength exercises. Finally, participants underwent the 1RM test; they were asked to perform one repetition with adequate technique throughout the range of motion, in the cadency 2:2 (2s in the concentric phase and 2s in the eccentric phase) until reaching the maximum load.23
At least 48h after the 1RM test, participants came back to the lab for the load calibration test (PHASE 2). On this day, the warm-up period included an organic phase on a cycle ergometer for 5min and then a local phase with a circuit of 1 set of 15 repetitions in each exercise at 40% 1RM. The calibration test consisted of performing 1 set of 12 repetitions in each exercise with an intensity between 70% and 80% 1RM.
After a minimum period of 24h from PHASE 2, the main phase of data collection was carried out (PHASE 3). Before starting the execution of the exercise session, participants underwent the same warm-up as in the previous phase. All the exercises were performed for 3 sets of 12 repetitions in a circuit format with the load obtained in the confirmation of the 1RM performed in PHASE 2 (70–80% 1RM). The passive rest interval between exercises was of 30–45s and between sets was 90–120s. Participants were asked to follow the cadence 2:2 during the exercise execution. Finally, PHASE 4 consisted of returning to the laboratory 24 after the end of the training for the acquisition of new thermal images. To standardize the effect of the menstrual cycle on thermoregulatory responses, the experimental protocol was carried out using the first day of menstruation as a baseline. Therefore, data collection started up to 72h after the start of menstruation.
The thermography protocol to register the body regions of interest (ROIs) was performed with a FLIR® (T420) imager, with a measuring range of −20 to +120°C, 2% accuracy, sensitivity ≤0.05°C, an infrared spectral band of 7.5–13μm, autofocus and a refresh rate of 60Hz and resolution of 320×240 pixels, setting the emissivity at 0.98. The first thermogram was taken at rest before starting the exercise session and the second thermogram was obtained approximately 20min after the end of the training. The thermograms were obtained in a room with controlled temperature (21.3±0.9°C) and humidity (59.2±2.4%), as recommended by Fernández-Cuevas et al.20 An anti-reflective black background was used behind the participant to minimize any reflection from infrared radiation.24 To obtain the thermograms, an acclimation period longer than 10min was considered.25
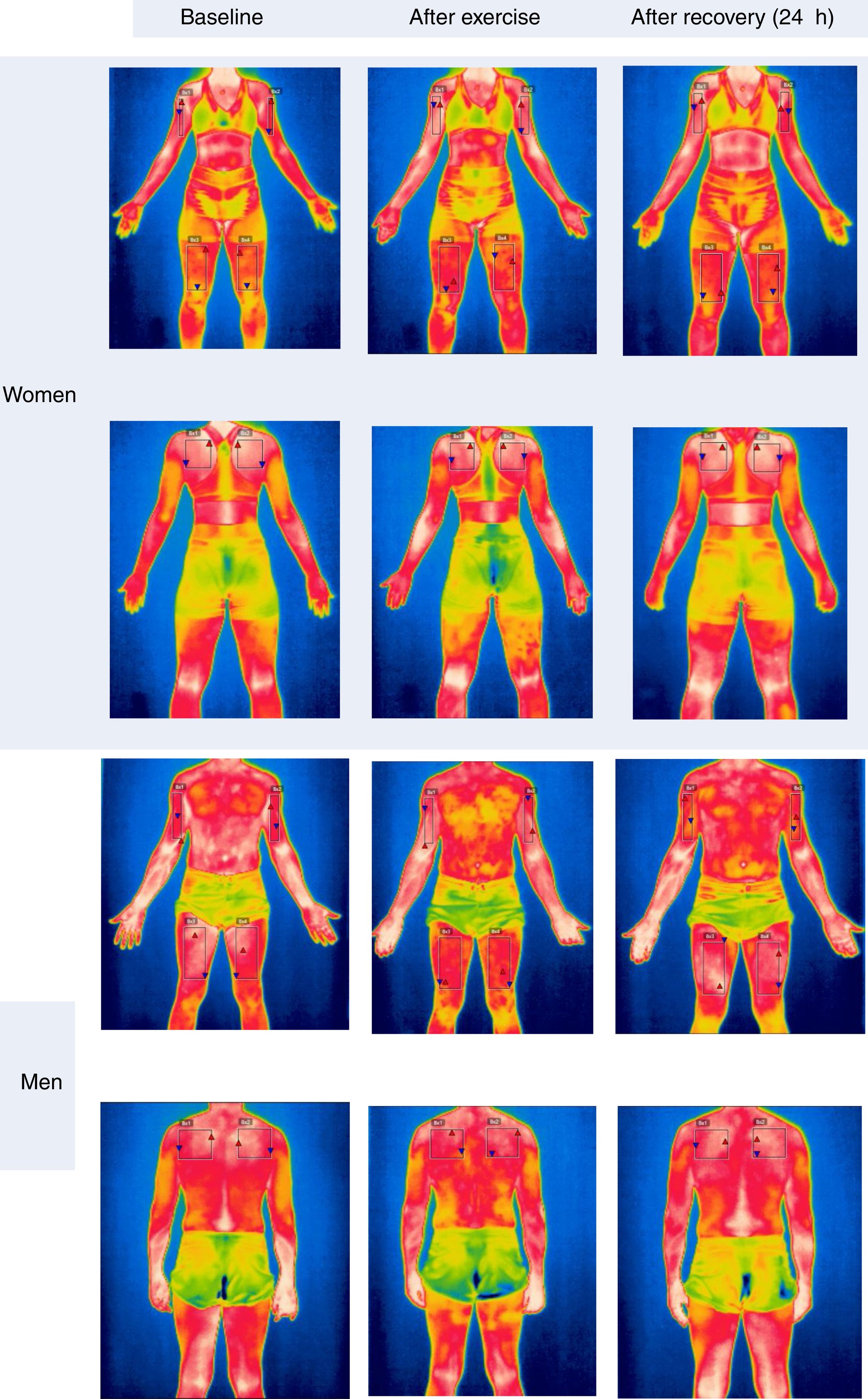
Two thermograms of the anterior and posterior views were obtained (Fig. 2). Through Flir Tools® 4.1 software and following the recommendations of Marins et al.,26 the ROIs considered in this study were delimitated in the thermograms by selecting the corresponding areas of the biceps brachii (between the axillary line and 5cm above the elbow), the upper back (over both scapulae) and quadriceps (between a point 5cm above the upper edge of the patella and occupying the greatest length of quadriceps).
Statistical analysisSince the Shapiro–Wilk test showed that the data were normally distributed, a descriptive statistic was used with mean, standard deviation, and maximum and minimum values. To analyze the temporal effect of exercise, ANOVA with post hoc Tukey's test was performed. The unpaired t-test was used to compare the thermal responses between men and women. Statistical analyses were performed in SPSS Statistics Subscription® version 22, setting the level of significance at α=0.05.
ResultsTable 2 shows the Tsk values of the three ROIs considered in this study for every participant, indicating the mean values on the right (R) and left (L) sides and asymmetries at baseline (Δ°C).
Baseline temperature values for participants in the three considered ROIs. R=Right; L=Left; S=Subject.
| S | Brachial biceps (°C) | Quadriceps (°C) | Upper back (°C) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| R | L | Δ°C | R | L | Δ°C | R | L | Δ°C | ||
| Men | S1 | 34.2 | 33.9 | 0.3 | 32.4 | 32.8 | 0.4 | 34.2 | 34.3 | 0.1 |
| S2 | 32.6 | 32.8 | 0.2 | 30.5 | 30.4 | 0.1 | 33.1 | 33.1 | 0.0 | |
| S3 | 32.8 | 32.8 | 0.0 | 33.0 | 33.4 | 0.4 | 32.8 | 32.6 | 0.2 | |
| S4 | 33.7 | 33.9 | 0.2 | 32.5 | 32.9 | 0.4 | 33.9 | 34.0 | 0.1 | |
| S5 | 32.8 | 32.8 | 0.0 | 32.3 | 32.4 | 0.1 | 33.7 | 33.9 | 0.2 | |
| S6 | 31.9 | 32.0 | 0.1 | 31.5 | 31.7 | 0.2 | 31.3 | 31.4 | 0.1 | |
| S7 | 33.3 | 33.2 | 0.1 | 31.4 | 31.9 | 0.5 | 32.5 | 32.1 | 0.4 | |
| S8 | 31.7 | 31.5 | 0.2 | 32.3 | 32.4 | 0.1 | 32.4 | 32.4 | 0.0 | |
| Women | S9 | 32.2 | 32.2 | 0.0 | 30.5 | 30.5 | 0.0 | 32.4 | 32.2 | 0.2 |
| S10 | 32.6 | 33.0 | 0.4 | 31.6 | 31.6 | 0.0 | 33.7 | 34.1 | 0.4 | |
| S11 | 31.5 | 31.7 | 0.2 | 31.4 | 31.3 | 0.1 | 32.5 | 32.7 | 0.2 | |
| S12 | 31.9 | 31.9 | 0.0 | 30.5 | 30.6 | 0.1 | 32.3 | 32.5 | 0.2 | |
| S13 | 31.7 | 31.9 | 0.2 | 30.8 | 31.0 | 0.2 | 32.8 | 32.9 | 0.1 | |
| S14 | 33.1 | 32.9 | 0.2 | 31.4 | 31.4 | 0.0 | 33.3 | 33.2 | 0.1 | |
| S15 | 30.4 | 30.2 | 0.2 | 30.6 | 30.5 | 0.1 | 32.2 | 32.2 | 0.0 | |
| S16 | 32.3 | 32.8 | 0.5 | 29.2 | 29.4 | 0.2 | 33.1 | 33.2 | 0.1 | |
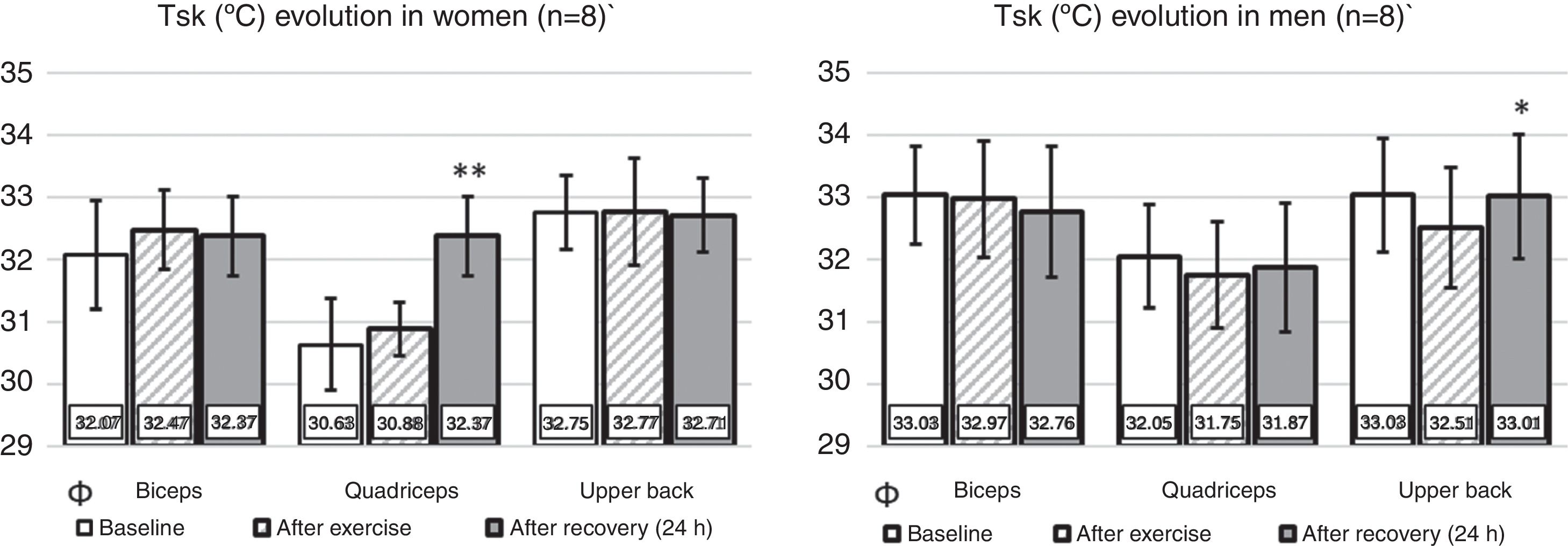
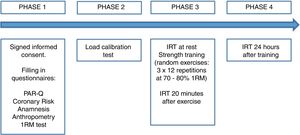
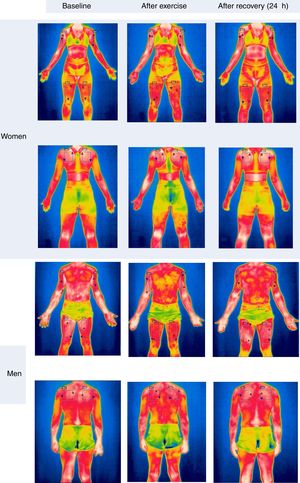
Since there was no significant difference between the contralateral ROIs, the arithmetic mean of the Tsk value between the right and left sides was considered for the statistical treatment at the three analyzed time points. Fig. 3 shows the mean Tsk values for every ROI; furthermore, Fig. 2 shows the thermogram evolution at three time points: rest (baseline), 20min after exercise, and 24h after exercise.
Behavior of Tsk (°C) in the three considered ROIs in the three analyzed moments. Ф Significant difference for the sex factor in basal condition. * Significant difference 24h after exercise compared to the values after exercise; ** Significant difference 24h after exercise compared to both basal and after exercise values.
At baseline, the Tsk of women was significantly lower than that of men (p<0.05). However, there were no significant differences between sexes both 20min and 24h after the end of the exercise (p>0.05). Regarding the time effect, there were no significant differences between Tsk values at baseline compared to the values obtained 20min after exercise both in men (p>0.05) and women (p>0.05). However, following 24h of recovery from resistance training, the upper back ROI showed a significant increase compared to the Tsk values measured 20min after exercise (p<0.05). In women, the quadriceps Tsk increased significantly during recovery compared to the baseline and 20min after exercise (p<0.05).
DiscussionBilateral thermal symmetry with differences lower than 0.5°C is one of the criteria to consider a state of thermal normality.26,27 The participants assessed in this study presented ideal initial conditions since there was no important asymmetry (>0.5°C) in the analyzed bilateral ROIs (Table 2), indicating the absence of injuries. Therefore, considering the thermal normality observed in the sample both in men and women, we decided to work with the arithmetic mean of the paired ROIs, similar to the approach previously used by Costa et al.28
Regarding the comparison of Tsk between the sexes at baseline, our data indicated that men had a significantly higher Tsk than women. This result agrees with other studies,26,28–30 which demonstrate that this thermal behavior is consistent. Tsk is higher in men due to metabolism, higher fat-free mass, and lower body fat percentage.26,29 In an investigation in which Marins et al.26 analyzed the thermal profile of men and women, there was a difference of approximately 1.0°C in the thighs Tsk (quadriceps and hamstrings), depending on sex. In our study, the greatest difference between the sexes was in the quadriceps ROI, with a mean difference of 1.42°C. This difference is probably related to higher body fat content in women, which can provide an insulating barrier to conductive heat flow to the skin and reduce its temperature. As shown in Table 1, body fat in women was approximately 4% lower than that in men.
On the other hand, the thermal profile was statistically similar between the sexes (p>0.05) both 20min and 24h after the end of strength training; therefore, there was a different behavior from that observed at baseline. Strength exercise may be a factor that interferes with this behavior.
Regarding the time effect of exercise, there is strong evidence that local thermal homeostasis changes during exercise,11,12 immediately after its completion,8 and during the recovery period of 24h after exercise.13,14,16 During aerobic exercise, the thermal response varies depending on whether the effort is carried out continuously or with progressive load.8,31–33 Immediately after exercise, the body thermal response may be influenced by two factors: the sweating evaporative effect and blood flow redistribution to the active muscles.34 After a 24-h recovery period, studies have found a local Tsk higher mainly in the body region previously stimulated in exercise, which would be related to anabolic activities of tissue repair and reestablishment of the energetic substrate.13,14,16
We hypothesized that Tsk would decrease significantly in all analyzed ROIs just after exercise and would increase during the 24-h recovery period. This behavior has already been reported immediately after running,31,33 cycle ergometer,12,35 and rowing exercises.8 It occurs due to higher activation of the process of evaporation of sweat, which reduces the Tsk, thus helping to control the internal temperature.1–3
However, the results found in the present study were not totally in this direction at the moment after exercise. There were no significant differences (p<0.05) in Tsk values after the specific strength training for both men and women. A possible explanation would be that the thermoregulatory adjustments may be different due to the characteristics of exercise (aerobic vs. strength). Aerobic exercise is performed continuously during a large time course, with a need for constant thermoregulation to maintain its intensity and with the engagement of large body mass during exercise. On the other hand, strength training is performed with rest interval and small and analytical muscle mobilization, which requires lower thermoregulatory demand and, consequently, lower sweat production.
Tsk is directly related to skin blood flow. During exercise, Tsk changes in accordance with the redistribution of blood flow from the inactive areas to the active areas.36 In long-duration and constant-load exercises, Tsk initially decreases, and subsequently, there is a Tsk increase followed by thermal maintenance.3 On the other hand, in short-duration and graded-load exercises, Tsk significantly decreases at the beginning of the exercise since there is a marked cutaneous vasoconstriction response to redirect blood flow to the metabolically active areas with every increment of load.3,31,32,37,38 The type of exercise in our study was performed through a circuit with 3 sets of 12 repetitions for every exercise. The intensity was constant throughout the session, respecting the protocol corresponding to 70–80% 1RM. This physical dynamic is different from the condition of previous studies since there is a very short recovery period between exercises. Therefore, this dynamic may have impacted the specific thermal adjustments in the resistance exercise protocol.
Fernández-Cuevas et al.34 observed that Tsk decreased immediately after a strength exercise compared to the baseline values, which is opposite to the results of the present study. One factor that may have influenced this result was the time needed to record the thermograms. In the study of Fernándes-Cuevas et al.,34 the thermograms were acquired immediately after the completion of the exercise; in the present study, this occurred just 20min after exercise. This interval time between the end of the exercise and thermogram acquisition can largely justify this difference from our results after exercise with those of similar studies.
In most of the studies analyzed in the literature review, all conditions of the data collection protocol were carried out in a laboratory11–13; thus, thermograms at rest, during exercise, immediately after exercise, and during recovery postexercise were obtained at the same place and with standardized environmental conditions. However, in the present study, there was a time interval of approximately 15–20min when the participants took a route from the gym to the laboratory room where the thermal camera was located. Therefore, the interval between the end of the exercise session and the data collection and the displacement of participants from the gym to the laboratory could have been enough to initiate the thermoregulatory adjustments, which would allow the restoration of the thermal profile similar to the baseline. This would indicate that the local thermal adjustments after performing the strength training are fast, as has already been reported in the recovery of the rest thermal profile after 30min of a jumping exercise session.39
Fig. 2 qualitatively shows the Tsk mapping of a man and a woman at rest, after exercise and 24h after training. It is possible to observe in the man that the upper back Tsk 24h after exercise is warmer (0.5°C) compared to the basal condition. On the other hand, the woman shows the upper back Tsk 0.3°C higher than the baseline value.
In the recovery period of 24h after exercise (Fig. 2), the quadriceps Tsk in women showed a significant increase compared to the baseline values, obtaining a mean difference of 1.74°C. This may indicate that, especially in women, there is still active local metabolic activity, which can facilitate tissue repair from training-induced muscle damage, as well as the recovery of energy from muscle glycogen depleted during training.15,16
During recovery from exercise, specific physiological adjustments are required to redistribute the blood flow, which may raise the local temperature due to high metabolic activity.13,14,34 This redistribution may be one of the factors responsible for the increments of Tsk observed during recovery after 24h, mediated by nitric oxide that acts as a microvascular vasodilator agent in the exercised area, causing an increase in local temperature. This response may be registered by IRT, which could be a justification for the Tsk increase 8h after a resistance training in the ROIs corresponding to the active muscles.34
The limitation of the study is the absence of measurements of internal temperature recorded through thermal pills. Moreover, the time required to collect thermograms just after the completion of the exercise was approximately 20min, which may have influenced the result. However, our results could indicate that thermal restoration after circuit-based resistance exercise is fast.
Future research should increase the number of strength exercises performed, change the range of repetitions and percentage of 1RM, and carry out data collection with a higher sample size to increase the statistical power of the results.
The main practical implication of our study is that the type of strength training performed (3 sets×12 repetitions at 70–80% 1RM) may induce thermal changes 24h after exercise compared to the baseline values in the exercised ROIs, which indicates that, from a thermal point of view, these areas would require a longer recovery time so that they can be stimulated again and thus reapply the prescribed training with the same level of performance.
ConclusionResting Tsk of women showed lower values than men in all analyzed ROIs. The strength training applied did not change the basal Tsk 20min after the end of exercise in the analyzed ROIs. However, 24h after training, the men's thermal profile presented significantly elevated Tsk in the upper back, and in women, the quadriceps region presented a significant Tsk increase compared to the baseline values.
Author's contributionVieira, SG: Acquisition of data, analysis and interpretation of results and drafting of manuscript.
Sillero-Quintana, M: Analysis of results and drafting of manuscript.
Silva, AG: Analysis of data and drafting of manuscript.
Marins, KO: Drafting of manuscript.
Marins, JCB: Planning, supervision, analysis and interpretation of results and drafting of manuscript.
Conflict of interestThe authors declare that they don’t have any conflict of interests.
Conselho Nacional de Pesquisa (CNPq).