Introducción: La fisiopatología de la tendinopatía rotuliana no es del todo conocida. La obtención de muestras clínicas en deportistas que permitan conocer la historia natural de la tendinopatía, sobre todo en las primeras etapas, es difícil. Por este motivo, el propósito de este estudio es crear, en una primera fase, un modelo experimental de tendinopatía rotuliana que simule la tendinopatía humana mediante la aplicación de colagenasa en tendón rotuliano en modelo de rata.
Material y métodos: El modelo experimental utilizado fueron ratas Wistar macho de 8 semanas de edad (N = 4). La administración de colagenasa se realizó, tras anestesia e inmovilización de los animales, mediante punción guiada por ecografía a nivel de la porción proximal y profunda del tendón rotuliano. La lesión tendinosa se evaluó 48 h después de la lesión mediante RMN tras lo cual se procedió a la eutanasia de los animales y extracción de los tendones rotulianos para su evaluación histológica.
Resultados: El modelo de lesión inducida con colagenasa demostró similitud a nivel de la histología con la tendinopatía rotuliana humana en la región de su inserción proximal.
Conclusiones: El modelo experimental de tendinopatía rotuliana en ratas induce la degeneración y distorsión de la arquitectura del tendón rotuliano en su porción proximal, situación similar a la observada en la tendinopatía rotuliana humana, y representa un excelente modelo preclínico para el estudio de nuevas terapias enfocadas al tratamiento de la tendinopatía.
Introduction: Patellar tendon pathophysiology is not still fully understood. The collection of clinical samples from athletes that could permit the analysis of the tendinopathy progression, especially in the early stages, is difficult. For that reason, the purpose of this study is to develop a new experimental animal model of patellar tendinopathy in rats which mimics the human tendinopathy by in vivo intratendinous collagenase injection in the proximal portion of the patellar tendon.
Material and methods: The experimental model used was 8-week-old male Wistar rats (N = 4). The administration of collagenase was performed by ultrasound-guided puncture at the level of the proximal and deep portion of the patellar tendon in anesthetized animals. The tendon lesion was evaluated 48 h after injury by magnetic resonance and then, the animals were euthanized and the patellar tendons were collected for histological evaluation.
Results: The collagenase-induced lesion model demonstrated important similarities with the human patellar tendinopathy in the region of the proximal insertion.
Conclusions: The experimental model of patellar tendinopathy in rat model induces a degeneration and distortion of the patellar tendon architecture in its proximal portion, which closely mimics to that seen in human patellar tendinopathy, and could represent an excellent preclinical model for the study of new therapies focused on treatment of tendinopathy.
Introduction
Patellar tendinopathy was first described by Blazina in 1973 as the ‘‘jumper’s knee’’.1 It is a frequent pathology affecting the proximal insertion of the patellar tendon and is one of the most common forms of chronic tendinopathy in athletes.2 Although numerous theories have been proposed, the causes and pathophysiology of patellar tendinopathy are still poorly understood.3,4
Cook and Purdam5 proposed a model based on the continuum of tendon pathology. This model considers 3 phases which may overlap and interconnect over time: reactive tendinopathy, tendon deterioration (failure of the healing process) and degenerative tendinopathy. This model proposes that the work load on the tendon plays an essential role in the onset and progression of tendinopathy.5 The findings commonly found in athletes are a degenerative tendinopathy, characterized by an increase in the non-collagen matrix (proteoglycans), mucoid degeneration with variable fibrosis, neovascularization, and an increase in the cellularity due to fibroblasts.6 On the other hand, Fu et al.7 also proposed a three-stage model characterized by an initial injury, a healing process and a failed clinical presentation. Despite the clinical importance of tendinopathy, its pathophysiology and clinical evolution are still poorly understood, which restricts the therapeutic interventions. The advance in the knowledge and understanding of the clinical evolution of tendinopathy presents important limitations, not only in the hitches to obtain tissue samples from human athletes but also due to these samples represent only the final stages of pathological processes with undisclosed onset and duration.8-10 For this reason, several animal models have been proposed for the study of tendinopathy, although their similarities with the human tendinopathy and their suitability to study the patellar tendon pathophysiology are still under controversy.11-13
Several animal models have been described in order to study the patellar tendinopathy, such as the surgically-induced tendon injuries,14-22 intratendinous collagenase injection,23-31 by administration of biological substances32,33 or induced by overuse,34-36 however, most of them are used for the testing of therapeutic approaches and only few are utilized to elucidate the pathological mechanisms of patellar tendinopathy. For this reason, the present work proposes the generation of a new animal model able to reproduce the patellar tendinopathy of the deep proximal portion that is commonly observed in athletes. This model could allow us to study and understand the pathological evolution of tendinopathy in its proximal portion, as well as to evaluate the effectiveness of new therapeutic interventions in order to promote the healing processes of patellar tendon.
Materials and methods
Animals
The experimental animal model used was 8-week-old male Wistar rats (Harlan). The total number of animals used was 4. The rats were maintained at 22-24 ¿C in a dark/light cycle of 12 h, with access to food and water ad libitum. All procedures were carried out in accordance with Spanish (Real Decreto 53/2013) and European (2010/63/EU) legislation and approved by the Departament d’Agricultura, Ramaderia, Pesca, Alimentació i Medi Natural del Gobierno Catalán (Generalitat de Catalunya).
Collagenase-based injury model
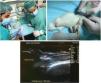
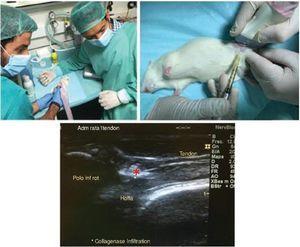
Animals were anesthetized by intraperitoneal injection of a mixture of ketamine (75 mg/kg) and xylazine (10 mg/kg) and placed in supine position facing up the left knee joint prior to the surgical procedures. The surgical process for the generation of patellar tendon injury is illustrated in Fig. 1. Knee joint was previously shaved and sterilized with 70% alcohol. The tendon injury was generated by ultrasound-guided injection of 10% collagenase solution dissolved in saline previously filtered for sterilization with a 0.22 μm filter (Nalgene), using a 29 G needle (0.33 mm inner diameter). The intervention was performed on the anterior side of the knee, inserting the needle into the proximal portion of the tendon until reaching the deep fibers. Once in the selected area, 20 μl of collagenase solution were released in the proximal tendon insertion area (Fig. 1). After the surgical procedure, post-surgical analgesia (buprenorphine 0.01 mg/kg) was administered subcutaneously to all animals. Our study followed the ethics standards in sport and research in sport sciences.37
Figure 1 Collagenase-based injury model. A dose of collagenase was administered in the proximal and deep portion of the patellar tendon by ultrasound-guided injection (*).
Magnetic resonance imaging analysis
In vivo 1H-magnetic resonance imaging (MRI) studies were performed at the Autonomous University of Barcelona (UAB, Barcelona, Spain) using a 7T Bruker BioSpec 70/30 USR (Bruker BioSpin GmbH, Ettlingen, Germany) system equipped with a mini-imaging gradient set (400mT/m) and using a quadrature transceiver volume coil with 72 mm inner diameter.
Rats were positioned in a bed, which allowed delivery of anesthesia (isoflurane, 1.5-2.0% in O2 at 1 L/min), with an integrated heat water circuit for body temperature regulation (Fig. 2). Body temperature was measured with a rectal probe and maintained at 37 ± 1 ¿C. Respiratory frequency was monitored with a pressure probe and kept between 60 and 80 breaths/min. Low resolution T2-weighted fast spin-echo images were initially obtained in axial, sagittal and coronal planes to be used as reference scout images. Imaging parameters for these images were: effective echo time (TEeff) = 36 ms; repetition time (TR) = 3 s; echo train length (ETL) = 8; field of view (FOV) = 6 × 6 cm2; matrix size (MTX) = 128 × 128; slice thickness (ST) = 2 mm; gap between slices (gap) = 0.5 mm; number of slices (NS) = 25 — axial, 10 — sagittal, 11 — coronal; number of averages (NA) = 1. High resolution T2-weighted fast spin-echo images were acquired afterwards in sagittal planes containing the lesion and the contralateral side. Experimental parameters for these images were: TEeff = 30 ms; TR = 4 s; ETL = 8; FOV = 3.2 × 3.2 cm2; MTX = 256 × 256; ST = 1 mm; gap = 0.1 mm; NS = 18; NA = 12; experimental time = 25 min 36 s. MRI data were acquired and processed on a Linux computer using Paravision 5.1 software (Bruker BioSpin GmbH, Ettlingen, Germany).
Figure 2 Anestesized animals were monitored for temperature and respiratory frequency during MRI analysis. High resolution T2-weighted fast spin-echo sagittal images were acquired of the injured and contralateral patellar tendons.
Histological analysis
After 7 days post-injury, the animals were euthanized by an intraperitoneal administration of ketamine (75 mg/kg) and xylazine (10 mg/kg) overdose. Tendons from both legs were immediately extracted from the patella to the tibia insertion including the Hoffa fat pad. Specimens were frozen in 2-methylbutane (Alfa Aesar, Johnson Matthey Company, Karlsruhe, Germany) previously supercooled in liquid nitrogen, and stored at −80 ¿C until used. The tendon samples were sectioned longitudinally (10 [H9262]m thick) using a cryotome (Leica Microsystems, Wetzlar, Germany) at −20 ¿C and mounted on PolylisineTM slides (VWR, Leuven, Belgium). Consecutive frozen tendon sections were used for histological analysis. Samples were stained with hematoxylin-eosin (1 min hematoxylin and 15 s eosin), washed in water and dehydrated with ethanol gradient solutions (1× 50% ethanol, 2× 70% ethanol, 2× 90% ethanol, 2× 100% ethanol, 1 min each) and cleared in xylene (5 s). After air drying, the slides were mounted with DPX medium and a coverslip (VWR, Madrid, Spain). Microphotographs were acquired using a BX-61 (Olympus) microscope equipped with a DP72 camera (Olympus) and CellSens® Digital Imaging software (version 1.9).
Results
MRI analysis of patellar tendon injury
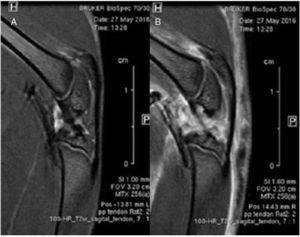
MRI sagittal images of healthy and injured patellar tendons were compared. No differences were detected in the alignment or thickness of tendon fibers along the tendon tissue. Diffuse edema affecting mainly the articular and muscular structures was observed (Fig. 3).
Figure 3 Comparative study using magnetic resonance imaging. No differences were found in the structure of the healthy patellar tendon (A) and in the injured tendon (B).
Macroscopic analysis of tendon injury
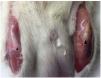
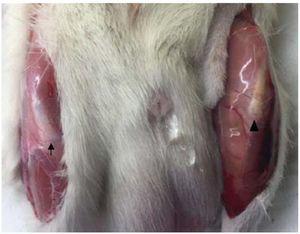
Clear macroscopic differences were found between healthy and injured tendons. Thus, healthy tendon showed a bright and intense white color and a strong consistency, whereas the injured tendon presented a yellowish brown color and a gelatinous presence. We observed that the peri-articular tissues, mainly the adjacent skeletal muscles, presented changes in color and consistency (Fig. 4).
Figure 4 Macroscopic analysis. Comparison of healthy (left) and injured tendon (right). The injured tendon has a yellowish-brown color and a soft gelatinous consistency (¿).
Histological analysis of patellar tendon
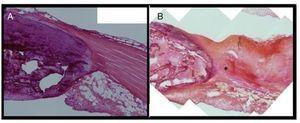
Contralateral healthy tendon showed a uniform appearance showing compact and well aligned collagen fibers. Tenocytes showed a spindle shape and localized in parallel to the tendon fibers pattern. The structure of Hoffa’s fat showed normal characteristics. In contrast, in the injured tendon a degeneration of the collagen fibre’s structure was clearly observed, showing also an evident corrugated structure and empty spaces between the adjacent fiber bundles at the level of the proximal third of patellar tendon. We also observed a partial fragmentation of tendon fibers at the site of injection. Likewise, a clear disorganization of the Hoffa’s fat pad tissue was detected, suggesting its affectation and disruption as a consequence of collagenase administration (Fig. 5).
Figure 5 Comparative histological study of the healthy tendon (A) and the injured (B). The injured tendon presents a degeneration of the collagen structure at the site where the puncture was performed (*).
Discussion
The main finding of the present study was the demonstration of the similarity of the structure alterations of the collagenase-treated rat patellar tendon, characterized by the disorganization of collagen fibers and adjacent structures, with respect to the patellar tendinopathy observed in the human clinics. These findings suggest that the administration of a single dose of collagenase by ultrasound-guided injection is effective to closely reproduce the structural changes in the integrity of the proximal region of patellar tendon of human patellar tendinopathy.
For the first time, we generated an animal model which mimics the tendon tissue degeneration in the most common anatomical site observed in the human patellar tendinopathy. Other animal models of tendinopathy have been designed to induce tissue degeneration in the middle third part of the patellar tendon through a surgically-created ‘‘window’’.14-22 This mechanism of injury does not reproduce the lesion detected in the human clinics since the surgically-created defects are generated in tendon areas different to the most common site where the tendon pathology occurs, so we hypothesize that the tendon repair mechanisms in these models does not reproduce the pathology of the tendon subjected to repetitive work loads.35,38 Our model is directed to imitate the degeneration of the proximal deep fibers since both the biomechanics and the relationship of the proximal patellar tendon region with the adjacent structures, mainly with the Hoffa fat pad, are important not only for the progression of tendinopathy but also in the tendon healing processes.39,40
Excessive tendon workload during intense physical training is considered the main cause of pathological degeneration.41 For that reason, training has been used as a model to induce patellar tendinopathy.36,42,43 The main disadvantage of this technique is based on the necessity to spend a long period of time for the training of the animals in order to develop the characteristic pathological changes of human patellar tendinopathy. In our opinion, it would be interesting to create a combined model of patellar tendon degeneration based on an initial collagenase-based injury by intratendinous injection in the proximal deep region followed by an intense physical training period in order to induce more rapidly the characteristic pathological degeneration of the patellar tendon. Previous data reported in Achilles tendinopathy models created by ultrasound-guided peritendinous collagenase administration suggest that the administration of collagenase may lead to tendinopathy in rats and that elastase may be also involved in the development of chronic tendinopathy.44
There are some limitations in our study. The first one is the small amount of animals (N = 4) used for the study. Second, the dose of collagenase should be optimized in order to exactly reproduce the characteristic changes in human patellar tendinopathy and prevent the collateral damage of adjacent structures to the tendon. Perucca Orfei et al.45 generated a model in rat Achilles tendon which closely reproduced the histological changes observed in human Achilles tendinopathy. They evaluated the effect of two doses of intra-tendinous delivered collagenase (1 and 3 mg/ml). Although both doses induced disorganization of collagen fibers and increased cellularity, the higher collagenase dose treatment was able to induce a greater neovascularization and fat degeneration, being also time-dependent changes. In our study, these changes were not observed since the time elapsed from collagenase administration to tendon analysis was shorter. In addition, Perucca Orfei et al. exposed the tendon by a longitudinal incision of the skin, whereas we used a minimally invasive technique based on ultrasound-guided collagenase administration in order to induce tendinopathy in the specific proximal deep fibers region of the patellar tendon with the minimal disturbance of the structures and tissues of the knee joint. Moreover, our analysis performed by magnetic resonance demonstrates that the effect of collagenase is not localized exclusively at the tendon level but could also affect periarticular structures, a situation which has not been discussed in previous studies.
In summary, our study suggests that the ultrasound-guided administration of collagenase solution in the proximal deep region of the patellar tendon could represent a consistent model to reproduce the degeneration and changes commonly seen in human patellar tendinopathy. This new model will also allow us to develop new therapies directed to the amelioration and healing of patellar tendinopathy as a previous step to initiate clinical trials in human patients.
Funding
No sources of funding were used to assist in the preparation of this article.
Conflict of interest
The authors declare that they have no conflicts of interest directly related to the content of this article.
Acknowledgements
The authors thank the Department de Cirurgia de la Facultat de Medicina of the Universitat Autonoma de Barcelona (UAB). At the time of writing, David Domínguez was a Ph.D. student at the UAB and this work was part of his doctoral dissertation performed at this department under the oversight and direction of Dr. Gil Rodas and Mario Marotta.
Received 22 December 2016;
accepted 11 January 2017
Available online 13 April 2017
∗ Corresponding author.
E-mail address: david.dominguez@fcbarcelona.cat (D. Domínguez).