Osteoporosis is characterized by a deterioration of bone microarchitecture, low bone mass and an increased risk of fractures affecting mainly menopausal women.1 The prevention of this disease at early ages of life is a determining factor in reducing the risk of fracture in the elderly.2 Several studies have explored the osteogenic effect of physical activity.3–7 Adolescents engaged in sports showed lower incidence of traumatic fractures.2 Moreover, adults engaged in non-professional sports in early life have higher BMD than their inactive peers, especially women.8 Particularly, sports involving high level impact from ground reaction forces are more osteogenic than non-weight bearing activities, like cycling and swimming. Elite water sports9 can lead to an osteoporosis risk situation when started at earlier ages, or at any case not improving the bone consolidation for the senescence. At the elite level, artistic swimmers (AS) underwent a greater amount of water training hours per week for many years: eight to ten pool sessions per week, with speed swimming and artistic swimming-specific skills. The effect this may have on the future development of osteoporosis has not been defined.10,11 To the best of our knowledge, studies regarding BMD in highly trained older women athletes in aquatic sports have not been explored thorough fully before.
Several research related to different sports has tested the positive effect of a plyometric jump training program on premenopausal women's bone,12 even when osteopenia is already established.13 A high-intensity, low repetition, short-lived, multidirectional jump program is also very effective in producing an osteogenic response, which can be introduced into daily life.14 It is important to find out jump programs that can counteract the potential negative consequences of non-osteogenic sport on bone health. The unprecedented worldwide confinement, caused by COVID-19, in which Spain has been one of the most affected-with severe rules governing confinement may have changed physical activity and sedentary habits due to prolonged stays at home.15
In an attempt to resolve these issues, ex artistic swimmers were included in a two –phases study (I) to determine the effect of being involved in elite artistic swimmers during their youth on bone mineral density (BMD), bone mineral content (BMC) and body composition (BC) compared with sedentary controls; and (II) to evaluate the effects of a jump program on bone mineral density and body composition in a group of ex-Artistic Swimmers.
MethodsThe present investigation includes two phases, first (I) a cross-sectional observational study that compares bone variables in ex artistic swimmers with sedentary controls and (II) a randomized controlled trial, pre-post intervention jump program in a group of ex artistic swimmers during the COVID-19 confinement.
Study design, protocol and informed consent forms were reviewed and approved by the Ethics and Clinical Research Committee of the Consorci Sanitari de Terrassa (CST) and met the requirements of the Declaration of Helsinki for research on human beings. Informed written consent was obtained from all participants included in the study.
SubjectsDuring the first part of the study, a total of 25 Caucasian ex artistic women swimmers were evaluated, aged 36-52 years and a group of 50 age-matched Caucasian sedentary participants, who served as the control group. The researchers contacted the swimmers participants thanks to the help of current artistic swimmers and coaches. The Centre for Technical Studies with Radioactive Isotopes (CETIR) randomly recruited the sedentary controls following the inclusion criteria of the study.
Inclusion criteriaFemale ex-artistic swimmers with more than 8 years practice in their sport specialty when they were young, a minimum of 5 years’ experience in high level competition (at national or international level), at least 10 hours of weekly training and at least 10 years since the end of the artistic swimming practice.
The sedentary controls had no formal training in any sport and did not reach the minimum physical activity recommended by the WHO, i.e. they practice less than 150 minutes/week of moderate physical activity or less than 75 minutes/week of vigorous physical activity.
Exclusion criteria1) diagnosis of any medical condition, 2) the use of any medication known to affect bone metabolism (corticosteroids, oral contraceptives, supplementation or other drugs), 3) any contraindications for sports practice and 4) abnormal (non recommended) regular food intake 5) more than 150 min/week physical activity practice during the period in witch they stopped artistic swimming.
Medical historyTo control possible confounding variables, the participants reported a questionnaire, including medication used, known diseases, menstrual cycle,11 stress fractures history, current dietary dairy products intake, vitamin and mineral supplementation, alcohol consumption16 and smoking. Smoking was classified as: (0) no, (1) yes. Alcohol consumption was classified as: (0) never, (1) 1-4 times/month, (2) more than twice a week. Dairy products intake: (1): <2, (2): >2. Sunbath was classified as: (1): <15min, (2): 15-30min, (3): >30min.
Physical activity assessmentPhysical activity patterns, training history, including years of active sport-specific training, total training hours per week and the age of onset of the sport specific training were documented. During their youth, they competed at national and international levels.
Current and Past physical activity during 15 years in ex artistic swimmers and in sedentary controls were collected using a questionnaire.17
Measurement of Bone Mineral Density (BMD) and body compositionThe BMD (g/cm2) and BMC (g) were assessed using dual energy X-Ray absorptiometry (DXA) “Lunar DXA TM GE Medical Systems, version 12.30”. Total body, lumbar spine (L1-L4) and femoral neck BMD were measured according to standard operating procedures, with a coefficient of variation of 0.91%, 0.608% and 0.87% respectively.
Whole body DXA also assessed body composition and the variables selected were arms, legs, trunk, ginoid and android regions and total fat mass (FM) and arms, legs, trunk, ginoid and android regions and total fat-free mass (FFM).
Dual-energy X-ray absorciometry is a medical imagining device which has become the method of choice for the measurement of body composition in athletes18 and it is the reference method for measuring total BMD and diagnosing osteopenia and osteoporosis.19 Specific age and sex references were used to calculate BMD Z-scores and T-scores.
Participants were measured using DXA twice: pre and post intervention jump program. Participants were in light clothing, barefoot and without jewellery or metal buttons. All subjects went to the toilet before test. The same technician took all measurements. Athletes were evaluated in supine position, with their feet in slight internal rotation.
Medical check-ups were conducted at the CAR-CST medical department and bone densitometries were performed at the FCB Medical Unit.
Exercise interventionIn phase II, we evaluated on the same group of ex-Artistic Swimmers the effects of 8-month intervention program on Bone Mineral Density and Body Composition. Subjects were randomly assigned to one of the both groups: countermovement jump (CMJ) group and non-intervention group. A CMJ session consisted on 4 sets of 4 CMJ combined with a reactive jump with 10 seconds’ rest between each set. This type of jump exceeded osteogenic thresholds to increase bone mass in premenopausal women in a previous study.20 Before starting the intervention program, trained research assistants ensured that each participant could correctly execute a set of four CMJ combined with a reactive jump. Participants were instructed to stand with feet shoulder-width apart with their arms raised above their head, to flex the knees and hips with arms “swinging” downwards and then jump upwards with arms “swinging” in the intended direction of travel to perform a maximal jump for height. Participants were cued to immediately jump vertically again after the initial jump landing and control the final second landing.
The intervention was divided into 2 levels. The first level lasted a month: 10 CMJ combined with a reactive jump, unidirectional, performed on a hard surface, twice a day, with at least 8 hours between sessions. The second level lasted 7 months: Participants performed 4 sets of 4 CMJ combined with a reactive jump with 10 seconds’ rest between each set, twice a day, with at least 8 hours between sessions, 6-7 days/week. In this second level, in order to increase multidirectional applied forces, a lateral or forward jump had to be made, before executing the next vertical CMJ.
The intervention compliance was checked using a weekly survey sent via e-mail and completed by participants, recording the number of jumps performed.
Previous to the training intervention, vertical ground reaction forces (GRF) were determined to provide a measure of the impact loading during exercise. GRF were collected using a force platform (model 9281EA, Kistler Instruments Ltd., Winterthur, Suiza) sampling at 1500 Hz. GRF were scaled to units of body weight. The peak of the vertical reaction forces (PVRF) and the impact load rate (ILR) of every jump was calculated and presented as the mean ± SD of the group. ILR was considered as the slope of the vertical reaction force between the initial floor contact and the peak of the vertical reaction force. To minimize risk of injury and maximize efficacy of the intervention, the jump training was performed wearing sport footwear, used a progressive intensity design and increasing jumping time slowly over the 8 months period.
The jump intervention was designed based on data from studies in adulthood women 21 and animals 22 showing that an “ideal” exercise prescription for bone health should include the following items: load the skeletal sites of interest, high-impact activity, result in dynamic strain, be “unusual” and include rest between loading cycles (10–15 seconds), sessions (8 hours) and blocks (several days).
Statistical analysesThe descriptive factors are presented as absolute and relative frequencies for the qualitative variables and with their mean and standard deviation for the quantitative variables. In phase I, qualitative variables, the chi-square test was used. The Shapiro Wilk Test was used to assess normality. The Shapiro-Wilk test showed that the sample was normally distributed. All the variables obtained p-values > 0.05. Student t Test has been used to compare means of the group variable (ex -elite AS and sedentary controls). In phase II, independent t tests were used to examine baseline differences between the CMJ group and the non-intervention group. Paired t tests were used to examine pre and post training bone variables (BMD and BMC) and lean mass and fat mass in both groups. In both cases the homogeneity of variances has been proved with the Levene test.
Effect sizes (ES) of the differences (Cohen's d) were also calculated. The magnitude of the differences was considered to be trivial (ES<0.2), small (0.2 ≤ ES <0.5), moderate (0.5 ≤ ES <0.8), and large (ES ≥ 0.8).
The level of statistical significance was set at 5%. Statistical analyses were performed using SPSS ver25 (Armonk, NY: IBM Corp.)
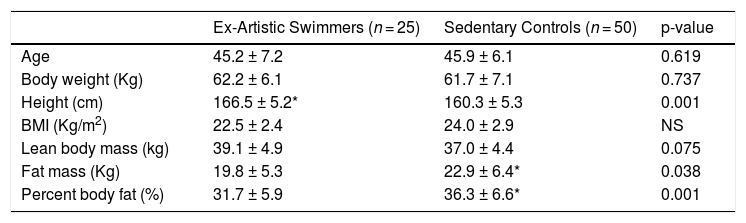
ResultsThe characteristics of the groups of the phase I are summarized in Table 1. There were no significant differences in age or body weight between groups. Artistic swimmers were taller (3.7%, ES= 1.192, p = 0.001); they had lower body fat percentage (4.6%, ES= 0.438, p = 0.001); and lower fat mass (13.6%, ES= 0.531, p = 0.038) than the sedentary control group.
Participant characteristics
| Ex-Artistic Swimmers (n = 25) | Sedentary Controls (n = 50) | p-value | |
|---|---|---|---|
| Age | 45.2 ± 7.2 | 45.9 ± 6.1 | 0.619 |
| Body weight (Kg) | 62.2 ± 6.1 | 61.7 ± 7.1 | 0.737 |
| Height (cm) | 166.5 ± 5.2* | 160.3 ± 5.3 | 0.001 |
| BMI (Kg/m2) | 22.5 ± 2.4 | 24.0 ± 2.9 | NS |
| Lean body mass (kg) | 39.1 ± 4.9 | 37.0 ± 4.4 | 0.075 |
| Fat mass (Kg) | 19.8 ± 5.3 | 22.9 ± 6.4* | 0.038 |
| Percent body fat (%) | 31.7 ± 5.9 | 36.3 ± 6.6* | 0.001 |
Values are presented as Mean± SD.
Artistic swimmers and age-matched controls were into peri-menopause and without oestrogen replacement therapy.
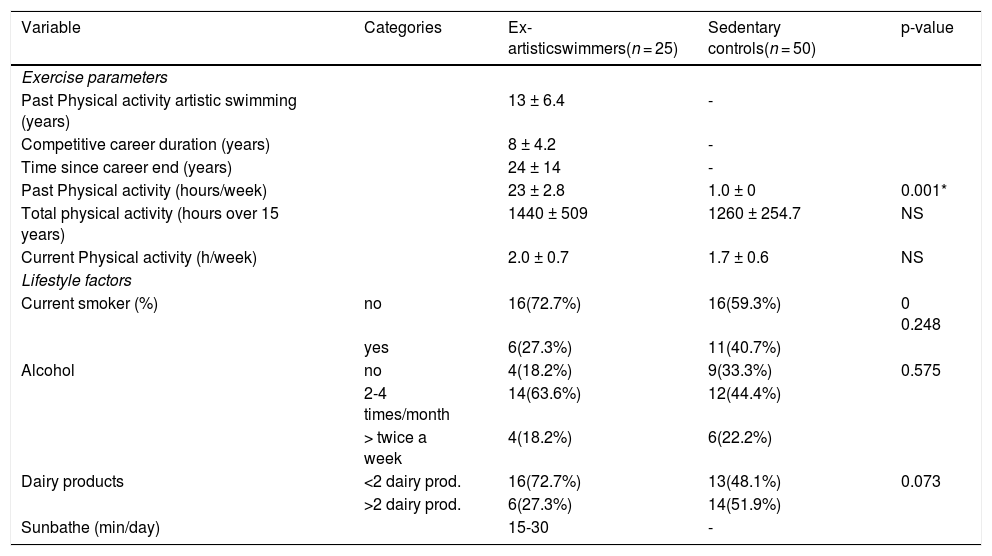
Table 2 shows physical activity levels for a period of more than 15 years. The artistic swimmers had significantly higher activity levels during their time of competition compared with the sedentary control group (p < 0.001). Water training was about 23 hours/week. The fitness routines included three-sessions/ week for 1 hour 30 minutes. Not all the swimmers continued to practice sport. The sports practiced since their retirement were Pilates, running, swimming; they were only active about 2 hours per week. The control group reported being active at gymnasium for about 1-2 hours per week. At the time of this study, there were no significant differences in activity levels between artistic swimmers and the sedentary control group. No significant differences in current life styles were found between groups.
Physical activity levels and life style at the time of study.
| Variable | Categories | Ex-artisticswimmers(n = 25) | Sedentary controls(n = 50) | p-value |
|---|---|---|---|---|
| Exercise parameters | ||||
| Past Physical activity artistic swimming (years) | 13 ± 6.4 | - | ||
| Competitive career duration (years) | 8 ± 4.2 | - | ||
| Time since career end (years) | 24 ± 14 | - | ||
| Past Physical activity (hours/week) | 23 ± 2.8 | 1.0 ± 0 | 0.001* | |
| Total physical activity (hours over 15 years) | 1440 ± 509 | 1260 ± 254.7 | NS | |
| Current Physical activity (h/week) | 2.0 ± 0.7 | 1.7 ± 0.6 | NS | |
| Lifestyle factors | ||||
| Current smoker (%) | no | 16(72.7%) | 16(59.3%) | 0 0.248 |
| yes | 6(27.3%) | 11(40.7%) | ||
| Alcohol | no | 4(18.2%) | 9(33.3%) | 0.575 |
| 2-4 times/month | 14(63.6%) | 12(44.4%) | ||
| > twice a week | 4(18.2%) | 6(22.2%) | ||
| Dairy products | <2 dairy prod. | 16(72.7%) | 13(48.1%) | 0.073 |
| >2 dairy prod. | 6(27.3%) | 14(51.9%) | ||
| Sunbathe (min/day) | 15-30 | - |
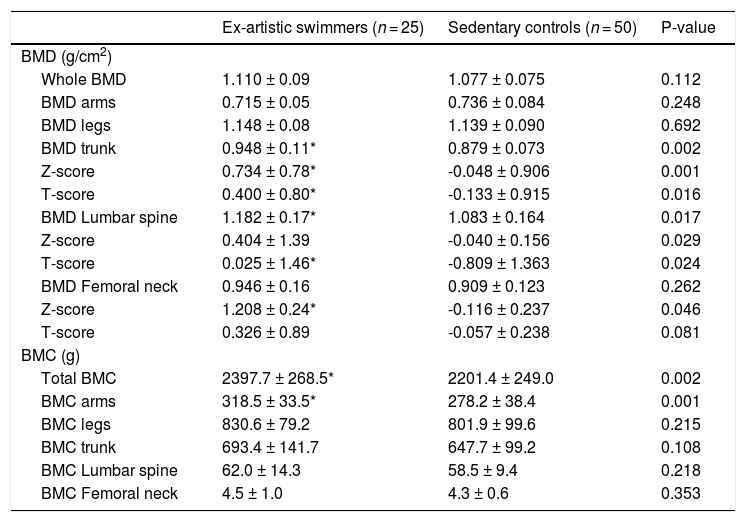
Table 3 shows regional BMD and BMC of the both groups. Z-score and T-score of total BMD (ES= 0.932; p = 0.001 and ES= 0.626; p = 0.016, respectively) and trunk's BMD (ES= 0.754; p = 0.002) in the artistic swimmers group were significantly higher compared with the sedentary group. Artistic swimmers had significantly higher Z-score of the femoral neck too (ES= 0.352; p = 0.046). No differences between both groups were found in total BMD, arms’ BMD and legs’ BMD. BMD of the lumbar spine was significantly lower (ES= 0.594; p = 0.017) in the control group compared with the artistic swimmers group. Further, the artistic swimmers had significantly higher total BMC (ES= 0.769; p = 0.002) and arms’ BMC (ES= 1.108; p = 0.001) than the control group. No differences were found in legs’ and trunk’ BMC.
Bone mineral density (BMD) and Bone mineral content (BMC) of the groups.
| Ex-artistic swimmers (n = 25) | Sedentary controls (n = 50) | P-value | |
|---|---|---|---|
| BMD (g/cm2) | |||
| Whole BMD | 1.110 ± 0.09 | 1.077 ± 0.075 | 0.112 |
| BMD arms | 0.715 ± 0.05 | 0.736 ± 0.084 | 0.248 |
| BMD legs | 1.148 ± 0.08 | 1.139 ± 0.090 | 0.692 |
| BMD trunk | 0.948 ± 0.11* | 0.879 ± 0.073 | 0.002 |
| Z-score | 0.734 ± 0.78* | -0.048 ± 0.906 | 0.001 |
| T-score | 0.400 ± 0.80* | -0.133 ± 0.915 | 0.016 |
| BMD Lumbar spine | 1.182 ± 0.17* | 1.083 ± 0.164 | 0.017 |
| Z-score | 0.404 ± 1.39 | -0.040 ± 0.156 | 0.029 |
| T-score | 0.025 ± 1.46* | -0.809 ± 1.363 | 0.024 |
| BMD Femoral neck | 0.946 ± 0.16 | 0.909 ± 0.123 | 0.262 |
| Z-score | 1.208 ± 0.24* | -0.116 ± 0.237 | 0.046 |
| T-score | 0.326 ± 0.89 | -0.057 ± 0.238 | 0.081 |
| BMC (g) | |||
| Total BMC | 2397.7 ± 268.5* | 2201.4 ± 249.0 | 0.002 |
| BMC arms | 318.5 ± 33.5* | 278.2 ± 38.4 | 0.001 |
| BMC legs | 830.6 ± 79.2 | 801.9 ± 99.6 | 0.215 |
| BMC trunk | 693.4 ± 141.7 | 647.7 ± 99.2 | 0.108 |
| BMC Lumbar spine | 62.0 ± 14.3 | 58.5 ± 9.4 | 0.218 |
| BMC Femoral neck | 4.5 ± 1.0 | 4.3 ± 0.6 | 0.353 |
Values are presented as Mean± SD.
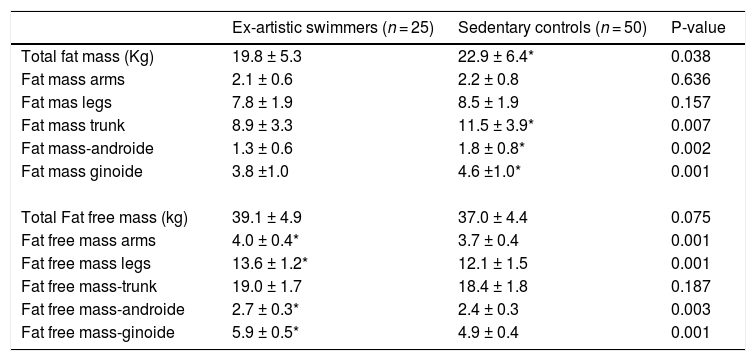
Artistic swimmers had lower total body fat mass (ES= 0.531; p = 0.038), trunk (ES=0.708; p = 0.007), ginoid (ES= 0.818; p = 0.001) and android regions (ES= 0.823; p = 0.002). Fat free mass was significantly lower in the control group (ES= 0.438; p = 0.075) with significantly differences in arms (ES= 0.844; p = 0.001), legs (ES= 1.068; p = 0.001), ginoid (ES= 2.055; p = 0.001) and android region (ES= 0.804; p = 0.003) (Table 4).
Segmental body composition parameters of the groups.
| Ex-artistic swimmers (n = 25) | Sedentary controls (n = 50) | P-value | |
|---|---|---|---|
| Total fat mass (Kg) | 19.8 ± 5.3 | 22.9 ± 6.4* | 0.038 |
| Fat mass arms | 2.1 ± 0.6 | 2.2 ± 0.8 | 0.636 |
| Fat mas legs | 7.8 ± 1.9 | 8.5 ± 1.9 | 0.157 |
| Fat mass trunk | 8.9 ± 3.3 | 11.5 ± 3.9* | 0.007 |
| Fat mass-androide | 1.3 ± 0.6 | 1.8 ± 0.8* | 0.002 |
| Fat mass ginoide | 3.8 ±1.0 | 4.6 ±1.0* | 0.001 |
| Total Fat free mass (kg) | 39.1 ± 4.9 | 37.0 ± 4.4 | 0.075 |
| Fat free mass arms | 4.0 ± 0.4* | 3.7 ± 0.4 | 0.001 |
| Fat free mass legs | 13.6 ± 1.2* | 12.1 ± 1.5 | 0.001 |
| Fat free mass-trunk | 19.0 ± 1.7 | 18.4 ± 1.8 | 0.187 |
| Fat free mass-androide | 2.7 ± 0.3* | 2.4 ± 0.3 | 0.003 |
| Fat free mass-ginoide | 5.9 ± 0.5* | 4.9 ± 0.4 | 0.001 |
Values are presented as Mean± SD.
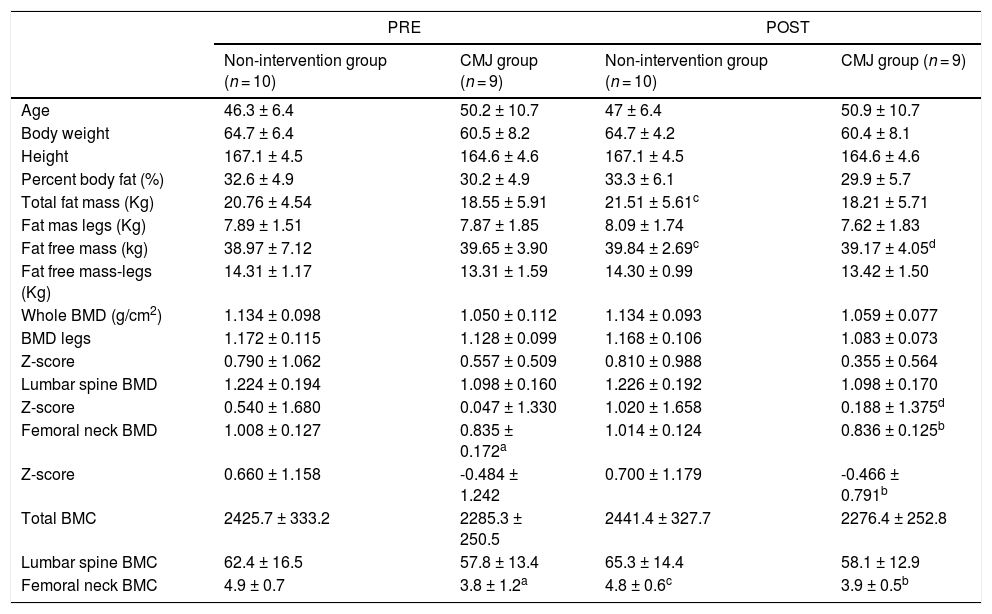
In phase II, sample size reduced to 19 participants due to personal reasons during the COVID-19 pandemic and two participants reported episodes of tibia periostitis due to jump training and didn't continue the study. The characteristics of the groups are summarized in Table 5. Subjects were randomly assigned to one of the both jumping groups during COVID-19 confinement: the CMJ group (n = 9) and the non-intervention group (n = 10). The intervention compliance was 92%.
Participant characteristics, Body Composition and Bone values of elite female ex-Artistic Swimmers (CMJ group and non-intervention group) PRE-POST intervention program.
| PRE | POST | |||
|---|---|---|---|---|
| Non-intervention group (n = 10) | CMJ group (n = 9) | Non-intervention group (n = 10) | CMJ group (n = 9) | |
| Age | 46.3 ± 6.4 | 50.2 ± 10.7 | 47 ± 6.4 | 50.9 ± 10.7 |
| Body weight | 64.7 ± 6.4 | 60.5 ± 8.2 | 64.7 ± 4.2 | 60.4 ± 8.1 |
| Height | 167.1 ± 4.5 | 164.6 ± 4.6 | 167.1 ± 4.5 | 164.6 ± 4.6 |
| Percent body fat (%) | 32.6 ± 4.9 | 30.2 ± 4.9 | 33.3 ± 6.1 | 29.9 ± 5.7 |
| Total fat mass (Kg) | 20.76 ± 4.54 | 18.55 ± 5.91 | 21.51 ± 5.61c | 18.21 ± 5.71 |
| Fat mas legs (Kg) | 7.89 ± 1.51 | 7.87 ± 1.85 | 8.09 ± 1.74 | 7.62 ± 1.83 |
| Fat free mass (kg) | 38.97 ± 7.12 | 39.65 ± 3.90 | 39.84 ± 2.69c | 39.17 ± 4.05d |
| Fat free mass-legs (Kg) | 14.31 ± 1.17 | 13.31 ± 1.59 | 14.30 ± 0.99 | 13.42 ± 1.50 |
| Whole BMD (g/cm2) | 1.134 ± 0.098 | 1.050 ± 0.112 | 1.134 ± 0.093 | 1.059 ± 0.077 |
| BMD legs | 1.172 ± 0.115 | 1.128 ± 0.099 | 1.168 ± 0.106 | 1.083 ± 0.073 |
| Z-score | 0.790 ± 1.062 | 0.557 ± 0.509 | 0.810 ± 0.988 | 0.355 ± 0.564 |
| Lumbar spine BMD | 1.224 ± 0.194 | 1.098 ± 0.160 | 1.226 ± 0.192 | 1.098 ± 0.170 |
| Z-score | 0.540 ± 1.680 | 0.047 ± 1.330 | 1.020 ± 1.658 | 0.188 ± 1.375d |
| Femoral neck BMD | 1.008 ± 0.127 | 0.835 ± 0.172a | 1.014 ± 0.124 | 0.836 ± 0.125b |
| Z-score | 0.660 ± 1.158 | -0.484 ± 1.242 | 0.700 ± 1.179 | -0.466 ± 0.791b |
| Total BMC | 2425.7 ± 333.2 | 2285.3 ± 250.5 | 2441.4 ± 327.7 | 2276.4 ± 252.8 |
| Lumbar spine BMC | 62.4 ± 16.5 | 57.8 ± 13.4 | 65.3 ± 14.4 | 58.1 ± 12.9 |
| Femoral neck BMC | 4.9 ± 0.7 | 3.8 ± 1.2a | 4.8 ± 0.6c | 3.9 ± 0.5b |
CMJ: countermovement jump
There was a significant difference between the non-intervention group and the CMJ group for femoral neck’ BMD in the pre intervention in favour of those that do not jump (ES=1.154; p = 0.023), which was maintained in the post intervention (ES=1.430;p = 0.006); and for femoral neck’ BMC in the pre intervention (ES=1.136;p = 0.032) and post intervention (ES=1.621;p = 0.008).After the intervention period, the non-intervention group presented a significant decrease in femoral neck’ BMC (ES=0.288; p = 0.019) and a significant increase in fat free mass (ES=0.138; p = 0.001) and in FM (ES=0.96; p = 0.001). Furthermore, CMJ group showed a significant increase in Z-score lumbar spine BMD (ES=0.422; p = 0.003) and a significant decrease in fat free mass (ES=0.130; p = 0.001).
During the jump assessment before starting the intervention program, significant differences were found between the reactive jump and the final landing in the PVRF (ES= 1.363; p < 0.001) and in the ILR (ES= 0.83; p = 0.002). Participants obtained an average of PVRF of 4.08 ± 0.90 BW with an ILR of 45.86 ± 17.08 BW/s in the reactive jump and an average of PVRF of 3.06 ± 0.71 BW with an ILR of 35.72 ± 11.33 BW/s in the final landing.
DiscussionThe main finding of the present study was that the long-term exposure to high level artistic swimming training at a younger age produced later significant increase in Z and T-score of Total BMD, Lumbar Spine BMD and Z-score of Femoral Neck BMD compared with controls of similar age and menopausal status. It is important to highlight that lumbar spine and femur measurements are two assessment measures according to the WHO classification for diagnosing osteopenia and osteoporosis 19 and specifically related to the most susceptible sites for osteoporotic fracture. The specific improvement in these areas is probably due to the fact that artistic swimming involves the execution of repeated exercises with an important participation of core and hip muscles.23 Previous studies have suggested a site–specific skeletal response to the type of loading at each BMD site.24 Tveit study25 shows that after 20 to 29 years of retirement from sport, former male soccer players had a higher whole and legs’ BMD than controls, similar to Mantovani study,8 in line to the present study for whole BMD. These results were related with the special biomechanical characteristics of soccer as changes of direction, speed, jump and kicks that offer additional mechanical stress in lower extremities, different from our artistic swimmers. In fact, a previous study conducted by us in young Olympic athletes, aquatic athletes (swimmers, artistic swimmers, water polo players) and non aquatic ones (football, volleyball, field hockey players)10 conclude that mean BMD values were highest in non aquatic sports athletes at all measured sites compared to aquatic athletes. However, all high level sports participants, aquatic and non-aquatic, had higher values than the sedentary controls, in lumbar spine and femur measures, in line with our current study in adult women.
Despite our positive results in lumbar spine and femoral neck, we were concerned about the bone health of the ex elite aquatic athletes. They showed similar results than the sedentary group in total BMD, arms’ BMD and legs’ BMD. In swimmers, low BMD accrual and/or increased bone resorption can occur silently over time, leading to osteoporosis without developing a stress fracture during their competitive years.26 The main factors that determine adult bone health are peak bone mass density at skeletal maturity and the rate of bone loss with advancing age; therefore, maximizing premenopausal BMD should be a critical strategy for the prevention of osteoporosis in this population.14
The artistic swimmers included in this study presented greater fat free mass (FFM) arms and legs and lower total and trunk fat mass (FM) than the control group, in line with the literature.8,24 Probably, these results are related to the fact that Artistic swimming training involves the execution of repeated exercises with a variety of movements of arms and legs to accelerate, decelerate, rotate, and turn to pull the body out of the water and even jump. Thus, artistic swimmers could develop a large muscle mass in extremities that increase bone mass in legs and femoral neck, a situation that occur in our population. On the contrary, other studies found greater FM in former soccer players and similar FFM than controls.25 Legs were more affected by sports participation probably because these body segments are more exposed to biomechanical ground reaction forces generated by the sport. As well as, fat free mass was the most relevant determinant of all-bone related outcomes. It is stated that FFM greatly influence BMD and BMC in adolescent women.27 Muscle strength applied to bone could be an important predictor of BMD,4,28 even in water sports. Sports participation in early life positively affects muscle mass gains, mainly in adolescence, and these gains in muscle mass could affect BMD through life, in line with these women athletes. The present study provided some evidence that participation in high-level aquatic sports during early life is not as adverse for the bone in adulthood as hypothesized in previous studies in swimmers.29–31 The discrepancy between swimmers and artistic swimmers could be attributed to training differences related to the greater mechanical loading from repetitive pushing against the pool wall in speed swimming,32 propulsive strokes and resistance against water30 and maybe also the related to training exercises out of the water.
As we have previously mentioned, one of the main factors that determine adult bone health is the rate of bone loss with age. Women are more susceptible to bone loss during menopause, mainly because of significant hormonal changes and a decline in exercise solicitation. Intervention approaches should aim to foster more positive attitudes to aging and retirement and promote physical activity at all stages in life.33,34 In line with our concern for the bone health of our retired artistic swimmers we designed phase II that matched with Covid-19 confinement. Results of the intervention program applied on the Phase II showed a decrease in femoral neck BMC’ in the non-intervention group. Furthermore, the CMJ group showed an increase in Z-score lumbar spine BMD’, in line with the literature.21 These improvements may be related to the high-impact tasks performed during the intervention program. The magnitudes of the PVRF (4.08 BW's) and the ILR (45,86 BW/s) for the reactive jumps after the first landing of the CMJ, exceed the defined osteogenic thresholds previously shown to improve bone mass at clinically relevant sites for premenopausal women (>3 BW's and 43 BW•s-1). In line with these results, previous studies with bouts of exercise designs (short duration, high-impact) similar to the present study found improvement in BMD on femoral neck.35–37 A recent study conducted by us showed that after 22 weeks of a jumping rope and whole body vibration program, only 20 min/day, twice week, the olympic artistic swimmers team increased lumbar spine and femoral neck BMD’ compared to the same group of athletes without intervention program.38 It has been difficult to follow the program during COVID-19 confinement, in line with a study that showed that the Spanish adult population decreased daily self-reported physical activity and increased sedentary time during COVID-19 confinement. The lockdown and social distancing caused by COVID-19 has influenced common health behaviour. Vigorous physical activities and walking time decreased by 16.8% and 58.2%, respectively, whereas sedentary time increased by 23.8%.15
The present study has several limitations. The first one is the relatively small sample size in phase I and phase II, determined by the small number of former elite artistic swimmers. Years ago artistic swimming did not have many practitioners. The difficulty in the recall bias from collecting information on physical activity levels from 15 years ago may exist. In phase II, the main limitation is the compliance with the intervention exercise program with the COVID-19 confinement.
On the other side, the present study has also several strengths. Our study is distinctive in the way that it presents lifetime bone trait evaluation in high level artistic swimmers, who trained regularly at a high intensity, volume and frequency, the perfectly standardized sport performed during early life, unthinkable in general population where everyone has dietary and exercise habits and different lifestyles. There are few studies in the literature that have taken into account the physical activity levels, bone variables and body composition of former elite artistic swimmers. The current study presents a high-intensity, low repetition, multidirectional jump program that could be introduced into daily life.
ConclusionProfessional sport participation during childhood and adolescence was positively associated with bone health in adulthood, even in aquatic sports, and specifically in artistic swimmers.
Short bouts of home exercise should be considered to reduce the risk of bone lost in retired artistic swimmers. After 8 months of high-impact jump training, BMD can be maintained or improved in premenopausal women.
Funding/support statementNo financial or material support of any kind was received for the work described in this article. No funding received.
Author's contributionAll authors contributed to the study conception and design. MB wrote the first draft of the manuscript and all authors commented on previous versions of the manuscript. MB carried out the bone densitometry studies, analysed the results and drafted the manuscript; FD conceived and coordinated this study, participated in its design and reviewed the draft; VF and EJ helped in the analyses of the results and reviewed the draft; LD participated in the bone densitometry studies; LG controlled the jump program and bone densitometry studies; VF and AT reviewed the document. All authors contributed to the editing and finalization of the manuscript. They have read and approved the final version of the manuscript, and agree with the order of presentation of the authors.
We thank all the ex elite female athletes who voluntarily accepted to participate as study subjects, all the technical staff of the Centre d'Alt Rendiment, as well as Manel Vela, head of the Sports Planification Department of the CAR, for his help in handling the study data. We also thank to Dr. Ramon Canal, Medical Director of the FC Barcelona Medical Services, the Consorci Sanitari de Terrassa and CETIR (Centre for Technical Studies with Radioactive Isotopes) for their unconditional support during the study development. We particularly thank Carmen Perez-Ventana for her help in bibliographic searches.