Spasticity is a common disorder in neurological patients. It causes an involuntary or sustained activity of the muscles and it is usually accompanied by weakness and loss of function. Resistance training could thus be an interesting therapeutic tool, so the objective of this review was to analyze the current literature on this type of exercise in spasticity, strength and function in this population. For this, a search was carried out in MEDLINE, CINAHL and SCOPUS and a total of 10 randomized controlled trials were obtained to carry out the analysis. In line with other research, it appears that resistance exercise does not worsen spasticity and could also improve the strength and functionality of these patients, so its practice under appropriate professional supervision is strongly recommended.
Spasticity is a common disorder affecting neurological patients with different pathologies such as multiple sclerosis, spinal cord injury or stroke, among others.1 Spasticity has been defined as “impaired sensorimotor control caused by an upper motor neuron lesion presenting with involuntary or sustained muscle activity”.2 It is associated with several clinical features such as exaggerated tendon reflexes, clasp-knife response and the appearance of pathological reflexes.1 This condition is associated with various complications such as falls, pain, pressure ulcers, etc.3 It is a disorder that mainly affects the anti-gravity muscles, so that in the upper limb the shoulder adductors, elbow, wrist and finger flexors, as well as the pronators of the forearm are usually affected. On the other hand, in the lower limb, the elevated muscle tone characteristic of spasticity predominates in the hip adductors, knee flexors, plantar flexors and inverters of the foot.1 Muscle tone in spasticity is related to the speed of movement, so the faster the stretch, the greater the resistance offered by the body segment.1 The consequences of spasticity range from a physical to a psycho-social aspect, so it hampers hygiene, sexual ability and other activities of daily living.1
On the other hand, strength training, refers to a specialised method of conditioning, which involves the progressive use of a wide range of resistive loads and a variety of training modalities designed to enhance health, fitness, and sports.4 It has been shown that well-programmed strength exercise can improve muscular strength, power, neuromuscular function, mobility, physical functionality and the performance of activities of daily living. It can also prevent falls, increase psychosocial well-being and preserve functional independence.5
The presence of spasticity and its severity have been linked to poor motor function.6-10 Current evidence suggests that the correct management of spasticity may improve motor function.11 Historically, spasticity was mainly treated with passive techniques as therapists suspected that active exercise might increase the condition.12 But this paradigm is changing due to growing scientific evidence that strength exercise appears to have no adverse effects on spasticity,12-17 and could be very beneficial for this patient profile, due to the benefits outlined above. Nevertheless, it is a difficult condition to address and the evidence for treatments is still limited. More research is needed on the management of the various treatments for spasticity.1 Therefore, the aim of this review was to analyse the effects of intervention with strength exercises and different muscle activations on spasticity, strength or function.
MethodsA literature search was conducted in February 2020 for publications registered in the MEDLINE, CINAHL and Scopus databases. The terms used in the search were the Medical Subject Headings (MeSH) descriptors “Muscle Spasticity” and “Resistance Training” as well as “spasticity” and “strength” as keywords. The search equations for each database are recorded in Table 1.
Explanation of the database search.
(MH, MeSh term; TITLE-ABS-KEY, tittle-abstract-keywords)
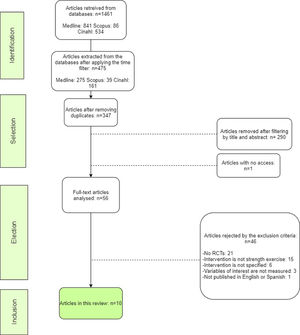
For the selection of results, they were filtered on the basis of certain inclusion and exclusion criteria set out in Table 2. The article selection process is also detailed in Fig. 1.
Inclusion and exclusion criteria.
To measure the methodological quality of the selected articles, the Jadad scale18 was applied. The results of this scale are detailed in Table 3.
Jadad scale.
| AUTHOR | Was the study described as randomized? | The method of randomisation was described in the paper, and that method was appropriate | Was the study described as double blind? | The method of blinding was described, and it was appropriate | Was there a description of withdrawals and dropouts? | Total score |
|---|---|---|---|---|---|---|
| Zhang et al.19 | YES | YES | NO | NO | YES | 3 |
| Jung et al.20 | YES | YES | YES | YES | YES | 5 |
| Yang et al.21 | YES | YES | NO | NO | YES | 3 |
| Simpson et al.22 | YES | YES | NO | NO | YES | 3 |
| Fernandez-Gonzalo et al.23 | YES | YES | NO | NO | YES | 3 |
| Bye et al.24 | YES | YES | NO | NO | YES | 3 |
| Qi et al.25 | YES | NO | NO | NO | NO | 1 |
| Coote et al.26 | YES | YES | NO | NO | YES | 3 |
| Gillett et al.27 | YES | YES | NO | NO | YES | 3 |
| Schranz et al.28 | YES | YES | NO | NO | YES | 3 |
The review process concluded with 10 valid results. The characteristics of these are shown in Tables 4 and 5.
Characteristics of trials with stroke patients.
(n, sample number; ♀, woman; ♂, man; AG, aquatic group; LG, land-based group; , average; L, losses; TENS, transcutaneous electrical nerve stimulation; EG, experimental group; CG, control group; NMES, neuromuscular electricular stimulation; TA, tibialis anterior; MG, medial gastrocnemius; ST, strength training; MST, mirror strength training; >, more than; MIVC, máximum isometric voluntary contraction; MAS, modified Asworth scale; EMG, electromyography; FAC; Functional Ambulation Category; BI, Barthel Index; CSS, Composite Spasticity Score; 10mWT, 10 meter Walking Test; TUG, Timed Up and Go; LHS, London Handicap Scale; BBE, Berg Balance Scale.)
Characteristics of trials with spinal cord injury, cerebral palsy and multiple slcerosis patients.
(n, sample number; ♀, woman; ♂, man; CG, control group; , average; L, losses; EG, experimental group; SCI, spinal cord injury; NS, not specified; CP, cerebral palsy; PRT, progressive reistance training; NMSE, neuromuscular electrical stimulation; ME, multriple slcerosis; HICT, high intensity circuit training; MIVC, máximum isometric voluntary; MAS, Modified Ashworth Scale; CSS, Composite Spasticity Score; GMFM, Gross Motor Function Measure; VAS, visual anaogic scale; BBS, Berg Balance Scale; TUG, Timed Up and Go; MPST, Muscle Power Sprint Test; TST, Timed Stairs Test; 6MWT, 6 Minutes Walking).
The intervention and results of each article reviewed are detailed below:
In the study by Zhang et al.19, both groups performed strength exercises with the same frequency and volume, i.e. 40 minutes per session, 5 times per week for 8 weeks. The aquatic group (AG) used water resistance for their exercises and the land-based group (LG) performed similar exercises but out of the water. The LG significantly improved knee flexion strength (p=0.035) and ankle dorsiflexion strength (p=0.042) compared to the pre-intervention measurement. On the other hand, the AG significantly improved strength in both knee flexion and extension movements (p=0.027 and 0.000 respectively) and in ankle plantiflexion and dorsiflexion (p=0.014 and p=0.036 respectively), that is to say, in all movements where they measured. The co-contraction ratio in knee extension also decreased in the AG, both compared to the first measurement and compared to the LG (p=0.000). The functional ambulation category (FAC) improved significantly in both groups, from 3 to 3.5 points in the LG (p=0.005) and from 3 to 4 points in the AG (p=0.004). The same is true for the Barthel Index (BI), whose score increased from 65 to 75 in the LG (p=0.000) and from 65 to 80 in the AG (p=0.000). This difference between groups was significant (p=0.009 in FAC and p=0.024 in BI). Spasticity, measured with the modified Ashworth scale (MAS) was not altered in either group.
In the trial conducted by Jung et al.20 both groups performed the same training, which consisted of squatting at the edge of a stretcher for 15 minutes, with the paretic leg further back than the healthy leg. This intervention was repeated 5 times a week for 6 weeks. In addition, they received one hour daily, also 5 days a week of conventional therapy. Prior to the above training, the experimental group (EG) received half an hour of TENS on the peroneal nerve at minimum sensory threshold. The control group (CG) had the electrodes placed but no current applied.
Patients in both groups improved significantly on all variables. The EG improved significantly more than the CG in spasticity, decreasing their score on the Composite Spasticity Score (CSS) from 11.5 points to 8.9 points (p=0.000), while the CG only improved from 11.9 to 10.8 points. There were also significant differences between groups, with the CG improving more in eyes open (p=0.013) and eyes closed (p=0.017) balance and hip strength (p=0.000).
Yang et al.21 conducted a study whose intervention consisted of 20 minutes of active contractions with the application of neuromuscular electrostimulation (NMES). One group trained the dorsal flexors, placing the electrodes on the tibialis anterior (TA) and another group trained the plantar flexors, placing the electrodes on the medial gastrocnemius (MG). After the contractions, they did 15 minutes of walking. This intervention was performed 3 times a week for 7 weeks. On the other hand, the CG received 20 minutes of stretching and passive and active mobilisations, followed by 15 minutes of walking.
The MG group had greater stride length in both legs (p=0.011 in the affected leg and p=0.028 in the healthy leg) compared to the pre-intervention measurement, but did not achieve greater strength in the plantar flexors. The TA group, on the other hand, achieved greater stride length of the affected leg (p=0.036), increased dorsiflexor strength (p=0.012), reduced MAS score from 2.4 to 1.5 points on average (p=0.028) and decreased spasticity index (p=0.025) compared to the pre-intervention measurement. This group also achieved significantly greater active plantar flexion during the toe-off phase of gait (p=0.015) and greater dorsiflexor strength (p=0.009) compared to the CG. The TA group's reduction in spasticity index was significantly lower compared to the MG group (p<0.05).
In the research carried out by Simpson et al.22, the intervention consisted of a warm-up with 1 minute of multiple dorsal flexions followed by 5 contractions at 50% of maximal voluntary contraction (MVC). During the main part of the training they performed 4 sets of 5 repetitions at maximum effort, in which they held each isometric contraction for 5 seconds on the healthy side. Between repetitions they rested for 5 seconds and between sets they rested for 3 minutes. The mirror strength training (MST) group performed the same training, but facing the mirror, strategically placed to look like the affected leg. The training was performed 3 times a week for 4 weeks.
Spasticity was significantly reduced (p<0.05) in all lower limb joints in both groups, from 1.64 to 1.14 at the hip, from 1.63 to 0.90 at the knee and from 1.83 to 1.07 at the ankle in the MAS in the strength training (ST) group and from 1.50 to 1.13 at the hip, from 1.50 to 0.73 at the knee and from 1.80 to 1.22 at the ankle in the MST group. The MST group achieved higher scores on the 10 meter Walking Test (10mWT) (p=0.000) and the London Handicap Scale (LHS) (p=0.030) compared to the pre-intervention measurement. There was no significant increase in strength of either limb in either group. There were no significant differences between groups in any of the variables measured.
In the study by Fernández-Gonzalo et al.23, the EG performed flywheel resistance training based on eccentric overload. They executed a leg-press exercise, completing 4 sets of 7 repetition maximums (RM). They rested 3 minutes between sets. The total volume resulted in less than 2 minutes of contraction per session. The intervention lasted for 12 weeks. The CG continued with their daily routine.
The results indicated that in the EG there were significant strength gains (p<0.05) in both the affected leg, which was the one they trained, and the healthy leg. The CG had a significant deterioration in the Berg Balance Scale (BBS), from 45.6 to 44 points. However, the EG improved significantly on this scale, increasing their score from 42.5 to 45.9 on average. The EG also achieved a significant improvement in the Timed Up & Go (TUG) test, from 20.3 seconds to 18.2 seconds. Spasticity was not significantly altered.
Bye et al.24 conducted a study in which the experimental group limbs performed 4 sets of 10RM. The intervention was performed 3 times a week for 12 weeks. The first two sets were isometric contractions and the last two sets were concentric contractions. The therapists exercised manual and maximal resistance in both isometric and concentric contractions. In case the physiotherapist did not have enough strength to resist, an external weight combined with manual resistance was applied. Both limbs received usual care, including walking, activities of daily living (ADLs) and if deemed appropriate by the therapists, treatments for pain, spasticity or contractures.
The authors had hypothesised that strength training would have no effect due to the patients' poor nerve conduction, however, strength increased significantly. The patients' perception of function and strength was measured on a 15-point scale where -7 means a change for the worse and 7 means a change for the better, through 0, which means no change. The perception was significantly increased compared to the control limb in both function (4.5 points for the EG and 2.4 points for the CG) and strength (4.7 points for the EG and 2.6 points for the CG). Spasticity did not increase or decrease significantly.
In the study that was conducted by Qi et al.25, the CG received NMES on the dorsal flexors for 20 minutes at motor threshold. They performed this 5 times a week for 6 weeks. The EG performed squats 10 times a day, for 2 minutes each time they did them. They also performed unspecified weight-bearing exercise, as well as walking up and down stairs.
Both groups improved significantly on every variable (p<0.05), but the improvement was significantly greater in the EG compared to the CG. The EG improved functionality on the Gross Motor Function Measure (GMFM) increasing the score from 44 to 70.6 on average. Gait speed also increased significantly compared to CG and spasticity decreased significantly from 12 points to 7.6 on the CSS.
Coote et al.26 conducted a trial in which both groups received the same training. This consisted of 6 lower limb exercises, which were squats, heel raises, step-ups, side-stepping, knee extensions and quadriceps contractions in the supine position. In all of them, weight was progressively added. They also progressed in volume, increasing from 1 set of 12 repetitions to 3 sets of 12 repetitions per exercise. They trained 2 days per week for the first 6 weeks and 3 days per week for the last 6 weeks, so the training lasted 12 weeks. The difference between groups was that the NMES group performed 4 of the 6 exercises with electrostimulation: squats, step-ups, knee extensions and quadriceps contractions in the supine position.
No significant differences were achieved between groups, but both groups obtained strength gains in hip extensors (progressive resistance training group (PRT); p=0.034 and NMES; p=0.019). Only the NMES group had gains in quadriceps strength (p<0.05) compared to the pre-intervention measurement and a significantly higher BBS score (p<0.001), increasing their score from 40 to 46 points. The PRT group also increased their score on this scale, starting with 38.5 and ending with 46.5 but this difference was not significant (p=0.059). In terms of TUG, the NMES group decreased from 21.1 to 18.1 seconds, but this difference was not significant, as was that of the PRT group, which decreased from 20.2 to 16.1 seconds. Spasticity with the visual analogue scale (VAS) remained without significant differences (PRT; p=1 and NMES; p=0.083).
Gillett et al.27 conducted a study in which the experimental group underwent strength training consisting of five lower limb exercises with progressively increasing load. After the strength training, they received functional anaerobic training of high-intensity activities such as stair climbing, directional changes and jumping over obstacles. The strength training volume was 70% of the total session. This intervention was performed 3 times a week for 12 weeks. The control group, on the other hand, did their normal daily activities.
In their hypothesis, the authors indicated that the subjects would gain strength and functional capacity. They significantly gained strength in the plantar flexors (p=0.025) but not in the dorsal flexors (p=0.088) compared to the CG. They also significantly improved, compared to the CG, in the Muscle Power Sprint Test (MPST) (p=0.026), in the “10×5m Agility Shuttle” test (p=0.016) and in the “6 Minutes Walking Test” (6MWT) (p=0.006), increasing the distance covered from 500.35 to 530.91 metres.
In the research conducted by Schranz et al.28, the PRT group performed progressive overload strength training consisting of 5 lower limb exercises. These exercises were adapted squats, heel raises, lunges, glute bridge and the lateral step-up. They performed 3 sets of 10 to 12 repetitions.
They also did a warm-up and a cool-down. On the first day, they calculated the 10RM in order to plan a good progressive overload. The high-intensity circuit training group (HICT) performed the same exercises as the PRT group but at a higher speed, without rest and as many repetitions as they were able. Both groups performed 3 sessions per week for 8 weeks.
The HICT group achieved significantly greater strength gains compared to the first measurement, but not compared to the PRT group (p=0.126). The HICT group significantly increased its MPST score compared to the PRT group. However, this latter one significantly improved the Timed Stairs Test (TST) over the HICT group (p=0.006). There were no significant changes in the 6MWT, as the distance increased very little in both groups, but the PRT group managed to significantly improve their TUG time from the first measurement, from 12.6 to 10.8 seconds. Both groups significantly improved functionality, although the PRT group scored significantly higher in functional tests such as TUG (p=0.05), decreasing the execution time from 10.3 to 9.1 seconds and TST (p=0.003). However, the HICT group achieved significant strength gains (p<0.05) compared to the EFP group.
DiscussionFeatures and sampleThis review is composed exclusively of RCTs, and therefore, taking into account their scores on the Jadad scale, in which all of them, except the study by Qi et al25, equal or exceed 3 points, it can be affirmed that the methodological quality of the studies in this review is good.
The aims of the studies are different, but they all analyse how a strength training intervention or different muscle activations, whether or not in combination with other treatment, would affect patients with spasticity. Exposure to strength training produces muscle strength gains.29 Moreover, in most of the articles included in this review that analyse the improvement in strength, increases in strength are obtained independently of the pathology of origin. The gain in strength in these patients is interesting because it has been found that muscle weakness correlates better with muscle function than spasticity30. It has also been observed that lower strength implies lower function31 due to a positive feedback loop, where muscle weakness will lead to poor function, which in turn will lead to disuse and this will aggravate the weakness again. This is why improving strength itself would be beneficial to this population.
In 5 of the 10 articles the pathology of the patients is stroke.19-23 In the remaining articles, the patients have cerebral palsy,25,27,28 spinal cord injury24 or multiple sclerosis.26 Although these are very different pathologies, all subjects in the studies have spasticity. Despite their aetiological differences, the results of this review show improvements in spasticity, strength and function regardless of the origin of the disorder. On the other hand, the mean sample size is 37.6 patients. It varies from 17 patients27 to 100 patients.24 Age varies greatly depending on the pathology, so that in studies where the sample includes a stroke,19-23 the mean age exceeds 50 years. However, those studies whose sample is made up of patients with cerebral palsy25,27,28 have a considerably lower mean age. Studies with patients with spinal cord injury24 and multiple sclerosis26 have a mean age of 46 and 52 years, respectively. In terms of the sex of the sample, most trials have more males than females. Different strength gains are usually associated with different hormone levels, and therefore sexual differentiation, but there are studies that suggest that relative strength gains are similar regardless of sex.32 In relation to losses, only the study by Coote et al.26 loses a relatively large number of participants, with 12 out of 37 subjects dropping out. However, in the rest of the studies there were very few or no losses.19-25,27,28
InterventionWhile in some studies in this review the control group receives no treatment or only usual care23,24,27, in most articles it receives some kind of intervention. This makes it difficult to compare between groups and also to compare between studies.
The frequency of training in the studies varies between 2 and 5 sessions per week and the duration of each intervention varies between 4 and 12 weeks. The exercises performed in each trial are also different. In all the articles they work with lower limbs and in the article by Bye et al.24 they work with both upper and lower limbs.
The interventions in the articles are not standardised, so each trial plans the training according to the criteria it considers appropriate, which makes it difficult to compare them. The selection of exercises is also highly variable, as some trials carry out an intervention with analytical movements such as dorsal or plantar flexion of the ankle21,22 and others carry out protocols with more complex movements such as squats or leg press.20,23,25 The remaining studies19,24,26-28 apply interventions that include other strengthening exercises. Regarding the training approach, trials should specify the variables of interest. However, studies such as Qi et al.25 or Jung et al.20 do not specify how many squat repetitions the subjects performed. Others, such as the trial by Yang et al.21, do not specify the intensity of the exercise performed. On the other hand, in the study by Bye et al.24 the physiotherapists exert a resistance on the participants that is supposed to be maximal, but this method has too much interpersonal variation and is not quantifiable, so it could lead to problems in terms of proper load dosing. Gillett et al.27 apply functional anaerobic training in addition to strength training. Although the latter accounts for more than 70% of the volume of the intervention, as is the case, it cannot be concluded that the results obtained were due to one type of training or the other, a limitation that the authors themselves point out in their research.
The fundamental pillar of strength training is progressive overload.33 However, only half of the studies carry out a progression of the load.23,24,26-28 The others perform the same protocol on both the first and the last day. In this way, they may achieve strength gains, but they are not optimising them.
Effects on spasticityIn relation to the results obtained, the most important finding is that spasticity did not increase significantly in any of the studies. In four articles spasticity remained the same,19,23,24,26 and in another four articles spasticity decreased.20-22,25
Two trials27,28 did not measure spasticity. Five19,21-24 of the articles used the MAS. In three of them19,23,24 there was no significant variation in spasticity, but in the studies by Yang et al.20 and Simpson et al.22 spasticity was significantly reduced. Only two articles20,25 used the CSS and in these two articles spasticity was significantly reduced. Coote et al.26 measured spasticity with a VAS in which patients marked on a 100mm line how much their muscle tone limited them in daily activities. Of all the methods they use to analyse spasticity, the MAS is the most widely used in the scientific literature, so its results should be considered the most important. These findings reinforce the previously stated idea that strength exercise does not increase spasticity. In this type of patient, neural control of movement is altered and there is both abnormal agonist contraction and abnormal antagonist inhibition34, which could reduce strength and thus functional capacity.35 Exercise, by proprioceptive neuromuscular facilitation, could improve this situation. In fact, some articles using electrotherapy as part of the treatment, such as Yang et al.20 state that the application of electrostimulation may not only increase strength, but may also reduce spasticity of the agonist or antagonist by relaxation induced after contraction or by reciprocal inhibition, respectively, as suggested by some studies.36,37
Efects on strengthAnother important finding is that in all the articles in which they measure strength, they achieve strength gains, except in Simpson et al.22 and Schranz et al.28. It is surprising that the latter case has not achieved strength gains, as their intervention is planned, with progressive overload, quite long lasting and at an enough intensity to achieve these gains. Since for a stimulus to be effective it has to exceed a certain threshold intensity,38 however, they did not achieve significant results. The case of Simpson et al.22 coincides with being the study with the shortest duration, 4 weeks, in which, although we know that strength gains can be achieved,29 it is possible that not enough time was intervened for the strength to increase. Most studies measure strength according to maximum isometric voluntary contraction.19-24,27 Two studies measured strength with a dynamometer, but did not specify how this was done.26,28 In the article by Zhang et al.19 it is likely that the land-based group achieved less strength than the aquatic group because the land-based group had lower intensity in several of the exercises they performed. The aquatic group took advantage of the resistance of the water and tried to perform the movement at the highest speed possible, so that the water applied the maximum resistance, but in the land-based group, it could be that some of the exercises were not very demanding. In this sense, the speed of execution is an important variable in strength training and is not taken into account in most studies. Thus, Fernández-Ortega et al.39 found that high-speed exercise, compared to low-speed exercise, can induce better neuromuscular adaptation in athletes, so this factor should be taken into account. This may be due to the fact that spasticity is speed-dependent, however, it might be interesting to study its effects.
Effects on functionIn terms of functional capacity, the studies measured it in different ways and with different tests. The most commonly used tests were the TUG, the BBS, the 6MWT and the MPST. In all the articles functionality is measured with more than one test and also in all of them there is improvement in some of the measurements they make. Comparison between studies becomes too complicated due to the low homogeneity of the functional tests.
The studies in which the BBS was performed23,26 obtained significant improvements in this scale. However, only one27 of the two studies27,28 using the 6MWT obtained improvements in it. In the four trials measuring TUG22,23,26,28, only two of them showed significant improvements23,28. However, in absolute numbers, the improvement of the article by Coote et al.25, which does not obtain significant improvements either in comparison with the first measurement or between groups, is greater than that of the article by Fernández-Gonzalo et al.23 (from 20.2s to 16.1s and from 20.3s to 18.2s, respectively). As for the MPST, it is used in two articles27,28 of patients with cerebral palsy and in both of them significant improvements in this test are obtained. On the other hand, it is possible that in the study by Coote et al.26 the NMES group obtained better results than the PRT group because they performed significantly more sessions (p=0.036) than the PRT group. Based on the review, it is logical to think that in all the studies the function of the patients increased in one way or another since in all but two studies22,28 strength increased. This could reinforce the previously discussed idea that strength gains and function are related.30,31
ConclusionIt can be concluded that strength training has no adverse effects on spasticity and it is advisable for this population to practice it due to its multiple benefits.
On the other hand, it must be taken into account that there is little standardisation in the measurement of the different variables. Therefore, it would be imprudent to draw strong conclusions about strength and functional gains in these patients. However, studying the results under caution, it seems that strength training and muscle activation increase strength gains in patients with spasticity, so that it could improve the functionality of these people. However, the current evidence is not yet conclusive about all the benefits that patients with spasticity might gain from strength training, so further research is needed to reach more firm conclusions.